A mammary fibroadenoma is a benign tumor consisting of a mixture of luminal epithelial and stromal cells, and sometimes admixed with myoepithelial cells. It is fairly common in cats and dogs [6]. However, spontaneous mammary tumors in goats, sheep, and horses are extremely rare [5], and a fibroadenoma has been reported only in one young Holstein cow [4] and a heifer [10]. To our knowledge, there are no reports of an ovine mammary fibroadenoma in the veterinary literature. We report the first case of mammary fibroadenoma in a young lamb.
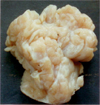
A 3-month-old, female Chios lamb presented with the progressive tumoral enlargement of the left udder since 1 month of age. The tumoral mass (15 × 13 × 13 cm) was well-circumscribed and was surgically excised and submitted for a histology examination. No recurrence was observed after surgery. Grossly, the cut surface of the mass was characterized by a rubbery consistency, whitish-grey lobular appearance with the lobules interlaced by thin fibrous septa (Fig. 1). The specimen was fixed in neutral-buffered 10% formalin from Baklan Veterinary Provincial Directorate, Denizli, Turkey and embedded in paraffin. Five micrometer thick sections were cut and stained with hematoxylin-eosin. Additional sections were stained with Masson's trichrome, periodic acid-Schiff (PAS) and toluidin blue methods and immunostained for their reactivity with the monoclonal antibodies against pancytokeratin-AE1/AE3 (Dako, USA), vimentin (Dako, USA), desmin (Dako, USA), α-smooth muscle actin (ASMA) (NeoMarkers, USA), estrogen receptor (Clone SP1) (NeoMarkers, USA) and progesterone receptor (Clone 1A6) (Novocastra, UK) using standard streptavdin-biotin peroxidase complex method (SBPC) with a commercial kit (Zymed, USA). The reaction product was visualized by 3, 3'-diaminobenzidine (DAB) chromogen (Zymed, USA) and counterstained with Harris' haematoxylin.
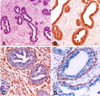
Microscopically, the mass consisted of proliferating fibroepithelial tissue, including well-differentiated ducts lined by whorls and interlacing bundles of abundant loose fibrovascular stroma (Fig. 2A). The ductal epithelium was composed of single or double layer of well-differentiated cuboidal to columnar epithelial cells settling down on a thin PAS-positive basement membrane. Some ductal structures included papillary projections to the lumen. Mitotic figures were rare in the epithelium. The stroma consisted of elongate to fusiform cells with oval nuclei, which were embedded into the eosinophilic extracelular matrix delineated by thin collagen fibers and infiltrated by some inflammatory cells including lymphocytes, macrophages and occasionally neutrophils.
Immunohistochemically, the ductal epithelium was positive for pancytokeratin (Fig. 2B), whereas stromal cells was positive for vimentin (Fig. 2C). Labeling for ASMA was strong in the basal cells of the ducts suggesting myoepithelial cells (Fig. 2D). Weak desmin staining was observed in only the smooth muscle of the vessel walls. There was no immunostaining for the estrogen and progesterone receptors.
A fibroadenoma is the main cause of an unilateral breast mass of teenagers and adolescents in humans [9], as well as the majority of mammary tumors (approximately 80-90%) in older female rats [7]. The fibroadenomas described in young human and young bovine cases usually show unilateral localization as in this case. The present case and bovine cases [4,10] are similar to that described in very young animals. This might be considered an aberration of normal development of the mammary tissue in herbivores.
The differential diagnosis of a mammary mass in children includes premature thelarche, asymmetric hyperplasia, fibroadenoma, phyllodes tumor, and lymphangioma abscess in human cases [2]. In the veterinary literature, the differential diagnosis of mammary fibroadenomas includes fibroepithelial hyperplasia, also known as fibroadenomatous hyperplasia or mammary hypertrophy, which occurs mainly in one or several mammary glands of very young cats treated with exogenous and endogenous progesterone [6] or estrogen [3]. Regression can occur after improving the hormonal status. A similar condition was also reported in a 2.5-year-old female goat, with no clinical aspects of the case provided [1]. Although the cases in cats and goats have been considered to be fibroadenomatous change, they are similar histologically to the fibroadenoma in human, canine and bovine cases.
Moreover, the fibroadenoma may be similar to a complex adenoma and benign mixed tumor of mammary gland in dogs and cats. A complex adenoma consists of both luminal epithelial cells and myoepithelial components. The latter cells form solid sheets and appear to produce a mucin like substance. A benign mixed tumor of the mammary gland is a tumor containing a mixture of epithelial, myoepithelial and mesenchymal cells as well as a combination of distinct cartilage, bone, fat and fibrous tissue [5,6]. Based on the histological and immunohistochemical findings, the present case is considered to be mammary fibroadenoma. The vimentin-positive spindle cells arranged concentrically around the tubules and vessels, and the ASMA-positive myoepithelial cells limited to only the basal cells of the ducts confirmed the periductal subtype of fibroadenoma. The tissues such as the bone and cartilage observed in benign mixed tumor were not observed in the present case.
An estrogen dependency has been suggested to play a role in the growth of fibroadenomas in humans, and estrogen receptor-beta is the only hormone receptor expressed by the stroma of fibroadenomas [8]. The immunohistochemistry for various hormone receptors including the estrogen and progesterone receptors in the present tumor was applied, but there was no positive signal for the estrogen and progesterone receptors. In any case, it was assumed that various hormones are include in the etiology of other benign or malignant mammary tumors in humans or animals and hormone receptors can simultaneously present in normal mammary glands from either unaffected or tumor bearing animals. Moreover, the benign and malign mammary tumors are positive for estrogen, progesterone or prolactin receptors to varying degrees [5]. Negative immunostaining for the estrogen and progesterone receptors may have resulted from a lack of cross-reactivity to the ovine species of antibodies used in this study.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download