Abstract
The comparison of the histologic healing and bronchopleural fistula (BPF) complications encountered with three different BS closure techniques (manual suture, stapler and manual suture plus tissue flab) after pneumonectomy in dogs was investigated for a one-month period. The dogs were separated into two groups: group I (GI) (n = 9) and group II (GII) (n = 9). Right and left pneumonectomies were performed on the animals in GI and GII, respectively. Each group was further divided into three subgroups according to BS closure technique: subgroup I (SGI) (n = 3), manual suture; subgroup II (SGII) (n = 3), stapler; and subgroup III (SGIII) (n = 3), manual suture plus tissue flab. The dogs were sacrificed after one month of observation, and the bronchial stumps were removed for histological examination. The complications observed during a one-month period following pneumonectomy in nine dogs (n = 9) were: BPF (n = 5), peri-operative cardiac arrest (n = 1), post-operative respiratory arrest (n = 1), post-operative cardiac failure (n = 1) and cardio-pulmonary failure (n = 1). Histological healing was classified as complete or incomplete healing. Histological healing and BPF complications in the subgroups were analyzed statistically. There was no significant difference in histological healing between SGI and SGIII (p = 1.00; p > 0.05), nor between SGII and SGIII (p = 1.00; p > 0.05). Similarly, no significant difference was observed between the subgroups in terms of BPF (p = 0.945; p > 0.05). The results of the statistical analysis indicated that manual suture, stapler or manual suture plus tissue flab could be alternative methods for BS closure following pneumonectomy in dogs.
Pneumonectomy is a lung resection technique that has been used to remove all lung lobes in humans and dogs when bilobectomy or lobectomy techniques are inadequate to remove the pathology in the hemi-thorax [12,13,23,32]. In dogs, pneumonectomy has been performed in some pathological conditions, such as lung tumors, congenital lung anomalies, chronic lung collapse, chronic progressive lung inflammation, post-traumatic diffuse parenchymal laceration, and bronchial rupture [12,23,25].
The reported morbidity and mortality rate after pneumonectomy is extremely high in humans suffering from respiratory, cardiac and gastrointestinal system problems, acute respiratory distress, pneumonia, pulmonary edema, pulmonary thromboembolism, bronchopleural fistula (BPF), pyothorax, esophagopleural fistula, cardiac herniation, lung lobe torsion, hemathorax and chylothorax [13,23,32]. The most commonly encountered complication of pneumonectomy in humans is BPF, which is described as a pathologic connection between the bronchus and pleural space [2,3,18,19,21,31-33,36]. Closure failure of the bronchial stump (BS) after partial or complete lung resection is the primary cause of BPF in humans, leading to a prolonged hospitalization period and the need for multiple operations [1,11,16-18,19,21,31-34,36]. Some authors [1,3,8] have reported of the incidence of BPF to be in the range of 0-28%, while others [19,21,31,32,34,35,37] have reported the incidence to be between 0% and 12%. The mortality rate of BPF has been reported to be between 15% and 70% [8,10,14,16,18-20,37]. Pneumonia, pleural infections and failure in BS closure are the primary risk factors for BPF [1,11,17,19,32]. The proper BS closure technique to prevent BS dehiscence after pneumonectomy has been long debated in the field of human thoracic surgery [3,17,31]. Therefore, the stapler technique has been used to prevent BPF in humans and dogs [1-3,17,22]. There seems to be no consensus between researchers regarding the optimal method for BS closure because several authors have made different suggestions for this procedure [1,6,8,11,19,21].
The surgical procedures for BS closure are focused on suture line (transversal versus longitudinal), suture technique (manual versus mechanical), clamping technique (open stump technique versus closed stump technique) and whether or not to wrap the BS (e.g. pleuralization, flap) [18,32].
Both manual (by running or interrupted suture) and mechanical sutures have been routinely used in humans and dogs [6,12,15,18,22,26,30]. The superiority of both methods has been clearly demonstrated by incidence of BPF complications in humans [1,18,20]. In recent years, the autogenic tissue wrapping of the BS after pneumonectomy has been reported to decrease the incidence of BPF [1,10,21,28,31,34]. Therefore, in this technique the intercostal muscle flaps are primarily used to support the vascularity of the BS and the line of anastomosis in the trachea. In addition, the popularity of intercostal muscle flap usage has been increased in human thoracic surgery due to their thickness, autogenous and self-vascularization properties [9,27,37].
In the present study, this literature data was compared with the histologic healing and BPF complications encountered following three different BS closure techniques (manual suture, stapler and manual suture plus tissue flab) after pneumonectomy in dogs were evaluated for a period of one month.
A total of 27 hybrid dogs (two years old, 20 kg in weight and sex was not considered) were used in this study. Histological healing was evaluated in 18 dogs (n = 18); nine dogs (n = 9) were reviewed separately due to postoperative complications. The 18 dogs were separated into two groups: group I (GI) (n = 9) and group II (GII) (n = 9). Right and left pneumonectomies were performed in the dogs in GI and GII, respectively. The dogs in GI and GII were further divided into three subgroups [subgroup I (SGI) (n = 3), subgroup II (SGII) (n = 3) and subgroup III (SGIII) (n = 3)] according to the BS closure technique performed. The main stem bronchus was closed with a manual suture in subgroup I (SGI) (n = 3), with a stapler in subgroup II (SGII) (n = 3), and with a manual suture plus tissue flab (pedicled intercostal externus-internus muscle) in subgroup III (SGIII) (n = 3) following pneumonectomy (Table 1).
The dogs were anesthetized and continued with 2% isofloran after the administration of xylasine HCl (1 mg/kg IM) and thiopental Na (15 mg/kg IV). Respiration was ensured by mechanical ventilation (15 ml/kg tidal volume, respiration rate 15/min and 25 cm H2O alveolar pressure).
All BS closures were carried out by the same surgical team as described previously. Thoracotomy incision was made on the 4th intercostal space according to the standard procedure in the SGI and SGII groups, but the 4th intercostal externus - internus muscle prepared as a pedicled flap in the SGIII group. The main stem bronchus was dissected after ligation of the pulmonary arteries and veins. Pneumonectomy was carried out using the open bronchi resection technique in groups SGI and SGIII. In SGII, however, resection was performed after stapler closure.
Subgroup I (manual suture): Vertical mattress sutures were applied over the excision line, and the end of the stump was sutured with a simple interrupted suture pattern using 2-0 vicryl (Ethicon, UK) (Fig. 1A).
Subgroup II (stapler): A TA-30 stapler (Ethicon, UK) (4.8 mm) was initially applied to the main stem bronchus, and the bronchus was then excised (Fig. 1B).
Subgroup III (manual suture plus tissue flab): Pedicled intercostal externus-internus muscle was extended towards the BS and then wrapped and sutured using a simple continuous suture pattern with 2-0 vicryl after manual suturing of the BS with 2-0 vicryl (Fig. 1C).
The pleural space was filled with warm sterile saline, and then 50 cm H2O endobronchial pressure was applied to determine the bronchial air leakage. Extra vertical mattress suture(s) was/were applied over the excision side of the BS in cases where air bubbles occurred due to the BS. Saline was aspirated, and a 28-French thoracostomy tube (Argyle, USA) was inserted into the pleural cavity ventro-cranially. The tube was fixed to the skin with a 'Chinese finger trap' suture pattern, and a Heimlich flutter valve was connected to the tube for pleural drainage.
Postoperative care was carried out in compliance with the rules of the National Society of Medical Research Principles of Laboratory Animal Care for a period of one month. The dogs were relieved with carprofen (5 mg/kg/d SC). Cefazolin Na (20 mg/kg IV tid) was used as an antibiotic agent for 5 days. Lactate ringer and hetastarch solutions were infused for postoperative fluid therapy. Hematologic parameters and blood gas analysis of the dogs were controlled routinely, and the dogs were closely monitored for suspected cardiac problems and respiratory failure after pneumonectomy. The dogs were checked radiologically in cases of suspected respiratory insufficiency. The thoracostomy tube and skin sutures were removed on days 5 and 7 after surgery, respectively. At the end of the postoperative first month, the dogs in GI and GII were sacrificed with high-dose thiopental Na. The BS was removed from each animal, fixed in 10% neutral-buffered formalin and then embedded in paraffin. Five micrometer thick sections from these samples were placed on slides and stained with hematoxylin and eosin for microscopic examination.
Statistical analysis was carried out using Fisher's exact test and Pearson's chi-square test to determine the differences between subgroups in terms of histological healing and BPF complications, respectively.
Postoperative ventrodorsal and lateral radiographs showed no signs of intra-thoracic pathology, such as pneumothorax or hemathorax, in the dogs during a one-month period following pneumonectomy. Mediastinal shifts were observed in the ventrodorsal radiographs of all the dogs (Fig. 2A); however, no abnormal clinical condition due to mediastinal shift was observed.
Complications were encountered in 9 of the 27 dogs (10 dogs in SGI, 7 dogs in SGII and 10 dogs in SGIII) and included two BPFs (on the 5th and 10th day), one postoperative cardiac failure (15th day) and one postoperative respiratory arrest (1st h after surgery) in the SGI group; one BPF (5th day) in the SGII group; and two BPFs (on the 1st week and 15th day), one cardio-pulmonary failure (on 3rd week) and one peri-operative cardiac arrest in the SGIII group (Table 2). Respiratory arrest and sudden onset of death were typically seen in the dogs with BPF. Tension pneumothorax and subcutaneous emphysema were observed in the postmortem radiographs of these animals (Fig. 2B). Necropsy of the dogs supported these findings and revealed bronchial dehiscence (Fig. 3) and atelectatic lung lobes.
In SGI, necropsy on the dog that died on the 15th day revealed myocardial petechial hemorrhagic spots related to the cardiac failure. In a different dog in SGI, the cause of the postoperative respiratory arrest (1 h after surgery) was insufficient lung volume, which caused the postoperative SPO2 value of the dog to decrease. In SGIII, alveolar emphysema, pneumonia and petechial myocardial hemorrhage were seen macroscopically in the dog with cardiopulmonary failure that died during the 3rd week after surgery. Peri-operative cardiac arrest after left pneumonectomy was seen as a complication in one of the dogs in SGIII. This was related to an underlying cardiac problem, which was determined to be an atrioventricular blockage and sinus pause after pneumonectomy based on the results of ECG.
Subgroup I (manual suture): Histological examinations revealed complete healing in 4 out of 6 bronchial stumps. Granulation tissue formation with new vessel formations as well as continued phagocytosis of the suture material was seen around these 4 bronchial stumps (Fig. 4A). Of the two remaining bronchial stumps, one had severe neutrophil infiltration, and the other had severe neutrophil infiltration along with purulent bronchitis. The healing rates of these two samples were considered to be incomplete.
Subgroup II (stapler): In the histological examination, complete healing was seen in 4 of these 6 bronchial stumps. New granulation tissues were seen around the BS and stapler particles were surrounded by macrophages and were being phagocytosed (Fig. 4B). There were no negative tissue reactions in the regeneration lesions. The other two samples had diffuse and severe hemorrhages in the regeneration area, and their healing was considered to be incomplete.
Subgroup III (manual suture plus tissue flab): Complete healing was observed in 3 out of 6 bronchial stumps. The muscle flap used for BS closure was degenerated and necrotized to a great extent, and a granulation tissue forming around the BS covered the area (Fig. 4C). Several siderocytes together with small hemorrhage areas were seen. The suture material was largely phagocytosed and phagocytic activity was in a continuum. In 2 of the 3 incomplete bronchial stumps, severe neutrophil infiltrations and severe hemorrhage were observed. The numbers of complete and incomplete histological healings and BPF complications within the subgroups are presented in Table 3.
There were no statistically significant differences in histological healing between the SGI and SGIII groups (p = 1.00; p > 0.05), nor between the SGII and SGIII groups (p = 1.00; p > 0.05). Similarly, there were no significant differences in BPF complications between the SGI, SGII and SGIII groups (p = 0.945; p > 0.05).
Respiratory, cardiac and gastrointestinal system complications are commonly observed after pneumonectomy in humans. The prevention and treatment of these complications are important topics in thoracic surgery. A considerable number of retrospective studies have been performed on bronchial closure techniques in human medicine, and a few experimental studies have been carried out on the basis of these retrospective studies [29,35]. However, BS closure techniques, their histological healing patterns and possible cardiac, respiratory and gastrointestinal system complications after pneumonectomy have not been compared experimentally and have only been reviewed in dogs [23].
Several techniques have been described for BS closure in humans [3,18,21,29]. Of these techniques, manual suture has been shown to be a safe and cost-effective technique [2,12,18]. The superiority of stapler suture to manual suture remains questionable, and several recent studies have compared the stapler with manual suture techniques [3,6,10,15]. Therefore, in the present study, manual and stapler suture techniques were used in the SGI and SGII groups, respectively. Manual suture, stapler and BS covering with flaps after manual suture closure are still in use today [3,6,8,12,18,22,23,29,30,33,34]. Pleural flaps, patch with pedicle, pericardial grafts, pericardial fat pad grafts, diaphragm, azygous vein, pericardiophrenic pedicles, mediastinum and transposition of extra-thoracic muscles (intercostal, serratus and latissimus dorsi) have been used as flaps in human thoracic surgery [4,5,21,29,31,34,35,37]. Intercostal muscle flaps are superior to other flaps due to their flexibility, thickness, vascularity and autogenous features, and the usage of these flaps has been recommended by a number of thoracic surgeons [9,27,37]. Thus, in this study, pedicled intercostal externus-internus muscle was used to buttress the manual suture line of the BS closure in the SGIII group.
There are many alternative suture techniques used for BS closure [3,6,12,18]. Hollaus et al. [16] advised the use of simple interrupted and over-to-over suture patterns for BS closure in humans. In veterinary literature, interrupted horizontal and continuous suture patterns have been used for BS closure [12,25]. In this study, hermetically horizontal mattress sutures were performed over the excision site, without affecting the vascularity of the main stem bronchus, and the end of the BS was manually sutured with a simple interrupted suture pattern in SGI. In humans and dogs, TA-55 and TA-30 staplers have been reported during pneumonectomy [14,30,31,33] by the parallel approximation of the mucosal membranous and cartilaginous portions of the bronchus and applying the staple transversally to the bronchus [15,21,25].
If the BS is to be closed with a suture, it is important that the same surgical team perform the procedure [2,3,18,21,30,32,36]. Therefore, in the present study, all BS closures were carried out by the same surgical team.
Current literatures indicate that the application of 20-40 cm H2O intra-bronchial pressure is sufficient to determine the amount of bronchial air leakage before thoracic closure [3,12,16,21,29]. Similarly, the intra-bronchial pressure increases by up to 200 mmHg in humans at the time of coughing [11]. Considering these findings and the possible postoperative coughing and barking of the dogs, 50 cm H2O pressure was applied intra-bronchially to test for air leakage. In cases of air bubbles occurring from the BS, extra vertical mattress suture(s) was/were applied over the excision side of the BS.
Antibiotic prophylaxis is recommended in clean-contaminated pulmonary surgery [24]. Pulmonary resection is associated with a considerable risk of infection; therefore, antibiotic prophylaxis has become a routine procedure during and after pulmonary operations [7]. Triple dose of antibiotic applications are advised in pulmonary surgery [3]. In addition, only one dose of prophylactic cephalosporin has been suggested to be an effective dosage following pneumonectomy [24]. In this study, cefazolin Na was administered during the postoperative period, and no infection-related complications were encountered.
Mediastinal shift is seen as a consequence of pleural cavity diseases [19]. This condition also results from pneumonectomy and causes esophageal and tracheal deviation [3,11,32]. Radiologically, the shifted mediastinum was observed in all dogs, and no complications from the mediastinal shift were seen for one month after the operation.
In humans, a history of fever and sudden onset of continuous coughing after lung resection raises the suspicion of BPF [3,19,32]. In this study, in contrast to the results in humans, the clinical findings in the dogs with BPF were respiratory arrest and sudden onset of death. Tension pneumothorax and subcutaneous emphysema were seen in the postmortem radiographs, and necropsy of the dogs revealed the occurrence of bronchial dehiscence and confirmed the BPF.
Possible cardiac, respiratory and gastrointestinal system complications after pneumonectomy have been reported in humans and dogs [23,29,35]. In this study, five BPFs, one cardiac failure, one postoperative respiratory arrest, one cardio-pulmonary failure and one peri-operative cardiac arrest were seen in the dogs (Table 2). Necropsy findings, postoperative SPO2 values and ECG findings revealed these complications in this study. It is our opinion that further studies can be planned to demonstrate the complications after pneumonectomy in dogs.
BPF is frequently encountered within the first three weeks after pneumonectomy in humans [8,19]. Pneumonectomy is considered to be an operative technique failure of BS closure [1,8,19]. In addition, the fifth postoperative day is the most critical day for bronchial dehiscence, and the incidence of early BPF in the suture technique is 8.6%, although its incidence is 1% in the stapler technique. The incidence of BPF following manual suture plus autogenic tissue coverage is 3.9% in humans [1]. The main focus of this study was not to determine the incidence of BPF in dogs, but we do report that we encountered five BPF complications in 27 dogs. All BPF complications were encountered within the first three weeks and resulted in death. There were no statistically significant differences between the techniques regarding BPF complication.
Information on the inflammatory reactions between the suture material and bronchi are important to the prevention of postoperative complications [31]. In the present study, neutrophil reactions with different intensities were seen after manual suture closure. In the present study, the stapler closure of the bronchi proved to be the preferred alternative within the three closure techniques used [21,31]. However, it is unclear which technique is superior to the others from a histological healing point of view [6]. It is important to note that avascular necrosis and inflammation of the BS after stapler closure have not been well explained in the literature [3,11,23,31]. In the present study, the stapler technique was better than the other techniques and resulted in less severe inflammation and a faster healing process. Hemorrhage was the only complication of the stapler technique. Current literatures indicate that vascular tissue with pedicle is required for the early healing of the BS [1,34,37]. In addition, pedicle flaps warranted the BS healing in the critical revascularization period continuing for 3-4 weeks, and decreased the risk of infection [4]. Self-vascularized flaps are reported to enhance the healing of the BS [1,34]. According to Algar et al. [1], intercostal flaps have shown excellent histological results. Although Yamamoto et al. [37] indicated that intercostal flaps prevented BPF complications, Demos [9] reported that these flaps could cause fibrosis, ossification and calcification. In our study, a severe inflammatory reaction was observed against the degeneration and necrosis of the flap and suture material. The inflammatory reaction caused a delay in the healing process. There were no statistically significant differences in histological healing among the subgroups. In the present study, we emphasize that the use of a thinner muscle flap can potentially reduce the inflammatory reaction, thus resulting in faster healing.
In conclusion, manual suture closure of the BS is still a current conventional technique. The usage of a stapler for BS closure is the best choice according to our histological data, although it cannot be considered to be a very economic technique. Manual suture plus intercostal externus- internus muscle flap technique has advantage for buttressing to BS. There were no statistically significant differences among the subgroups with regard to BPF complications. According to the histological healing results, the manual suture technique did not have an apparent advantage over the other techniques, but stapler closure seemed to be more acceptable than the other methods of closure. The use of manual suture plus tissue flap technique could not be recommended due to the observation of intense inflammatory reactions following this procedure. Statistical data indicated that none of the techniques seemed to have any superiority over the others with regard to histological healing. Taking the results of the statistical analysis into consideration, all three techniques (suture, stapler and suture plus tissue flab) can be used for BS closure.
Figures and Tables
Fig. 1
(A) Suture closure of the main stem bronchus (arrow) with 2-0 vicryl following pneumonectomy. (B) Application of a TA-30 stapler to the main stem bronchus after pulmonary artery and vein ligation. (C) Transposition and suturing of the 4th intercostal muscle flap (arrow) to the main stem bronchus following pneumonectomy.
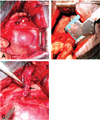
Fig. 2
(A) Mediastinal shift observed on the 15th postoperative day on a ventrodorsal radiograph of a dog with right pneumonectomy. h: heart. (B) This postmortem ventro-dorsal radiograph shows tension pneumothorax and subcutaneous emphysema (arrow) in a dog with BPF.
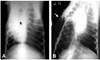
Fig. 3
Bronchial dehiscence as a necropsy finding of a dog with BPF. bs: bronchial stump, m: pedicled intercostal externus- internus muscle.
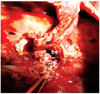
Fig. 4
(A) Granulation tissue formation and continuing phagocytosis of the suture material in subgroup I (manual suture). (B) Granulation tissue around the BS and stapler particles surrounded by macrophages in subgroup II (stapler). (C) Granulation tissue forming around the BS covered with muscle tissue in subgroup III (manual suture plus tissue flab). gt: granulation tissue, m: muscle tissue, mc: macrophages, s: suture material, sp: stapler particles, arrow: new vessel formation. H&E stain, ×200 (A&C), ×100 (B).

Table 1
Distribution of operations in groups evaluated histologically, and the number of complications and operations in each subgroup
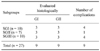
Table 3
Numbers of complete and incomplete histological healings and BPF complications in each subgroup*

*Abbreviations are the same as Table 1.
References
1. Algar FJ, Alvarez A, Aranda JL, Salvatierra A, Baamonde C, Lopez-Pujol FJ. Prediction of early bronchopleural fistula after pneumonectomy: a multivariate analysis. Ann Thorac Surg. 2001. 72:1662–1667.

2. Al-Kattan K, Cattalani L, Goldstraw P. Bronchopleural fistula after pneumonectomy with a hand suture technique. Ann Thorac Surg. 1994. 58:1433–1436.

3. Al-Kattan K, Cattelani L, Goldstraw P. Bronchopleural fistula after pneumonectomy for lung cancer. Eur J Cardiothorac Surg. 1995. 9:479–482.

4. Al-Kattan KM, Breach NM, Kaplan DK, Goldstraw P. Soft-tissue reconstruction in thoracic surgery. Ann Thorac Surg. 1995. 60:1372–1375.

5. Anderson TM, Miller JI Jr. Use of pleura, azygos vein, pericardium, and muscle flaps in tracheobronchial surgery. Ann Thorac Surg. 1995. 60:729–733.

6. Asamura H, Kondo H, Tsuchiya R. Management of the bronchial stump in pulmonary resections: a review of 533 consecutive recent bronchial closures. Eur J Cardiothorac Surg. 2000. 17:106–110.

7. Boldt J, Piper S, Uphus D, Fussle R, Hempelmann G. Preoperative microbiologic screening and antibiotic prophylaxis in pulmonary resection operations. Ann Thorac Surg. 1999. 68:208–211.

8. De Perrot M, Licker M, Robert J, Spiliopoulos A. Incidence, risk factors and management of bronchopleural fistulae after pneumonectomy. Scand Cardiovasc J. 1999. 33:171–174.

10. Dziedzic D, Orlowski TM, Jakimiuk R. Experimental study of the effects of different stapling devices in healing of the mechanically sutured bronchial stump. Eur J Cardiothorac Surg. 2000. 17:111–116.

11. El-Gamel A, Tsang GM, Watson DC. The threshold for air leak: stapled versus sutured human bronchi, and experimental study. Eur J Cardiothorac Surg. 1999. 15:7–10.

12. Fossum TW. Small Animal Surgery. 2002. 2nd ed. St Louis: Mosby;760–770.
13. Groenendijk RP, Croiset van Uchelen FA, Mol SJ, De Munck DR, Tan AT, Roumen RM. Factors related to outcome after pneumonectomy: retrospective study of 62 patients. Eur J Surg. 1999. 165:193–197.

14. Hakim M, Milstein BB. Role of automatic staplers in the aetiology of bronchopleural fistula. Thorax. 1985. 40:27–31.

16. Hollaus PH, Huber M, Lax F, Wurnig PN, Bohm G, Pridun NS. Closure of bronchopleural fistula after pneumonectomy with a pedicled intercostal muscle flap. Eur J Cardiothorac Surg. 1999. 16:181–186.

17. Hollaus PH, Setinek U, Lax F, Pridun NS. Risk factors for bronchopleural fistula after pneumonectomy: stump size does matter. Thorac Cardiovasc Surg. 2003. 51:162–166.

18. Hubaut JJ, Baron O, Al Habash O, Despins P, Duveau D, Michaud JL. Closure of the bronchial stump by manual suture and incidence of bronchopleural fistula in a series of 209 pneumonectomies for lung cancer. Eur J Cardiothorac Surg. 1999. 16:418–423.

19. Khan JH, Rahman SB, McElhinney DB, Harmon AL, Anthony JP, Hall TS, Jablons DM. Management strategies for complex bronchopleural fistula. Asian Cardiovasc Thorac Surg. 2000. 8:78–84.

20. Klemperer J, Ginsberg RJ. Morbidity and mortality after pneumonectomy. Chest Surg Clin N Am. 1999. 9:515–525.
21. Klepetko W, Taghavi S, Pereszlenyi A, Birsan T, Groetzner J, Kupilik N, Artemiou O, Wolner E. Impact of different coverage techniques on incidence of postpneumonectomy stump fistula. Eur J Cardiothorac Surg. 1999. 15:758–763.

22. LaRue SM, Withrow SJ, Wykes PM. Lung resection using surgical staples in dogs and cats. Vet Surg. 1987. 16:238–240.

23. Liptak JM, Monnet E, Dernell WS, Rizzo SA, Withrow SJ. Pneumonectomy: four case studies and a comparative review. J Small Anim Pract. 2004. 45:441–447.

24. Naunheim KS. Postoperative care and monitoring. Chest Surg Clin N Am. 1999. 9:501–513.
25. Nelson AW, Monnet E. Slatter D, editor. Lungs. Textbook of Small Animal Surgery. 2003. 3rd ed. Philadelphia: Saunders;396–398.
26. Pavletic MM. Surgical stapling. Vet Clin North Am Small Anim Pract. 1994. 24:395–412.
27. Philippi D, Valleix D, Descottes B, Caix M. Anatomic basis of tracheobronchial reconstruction by intercostal flap. Surg Radiol Anat. 1992. 14:11–15.

28. Rendina EA, Venuta F, Ricci P, Fadda GF, Bognolo DA, Ricci C, Rossi P. Protection and revascularization of bronchial anastomoses by the intercostal pedicle flap. J Thorac Cardiovasc Surg. 1994. 107:1251–1254.

29. Roberson LD, Netherland DE, Dhillon R, Heath BJ. Air leaks after surgical stapling in lung resection: a comparison between stapling alone and stapling with staple-line reinforcement materials in a canine model. J Thorac Cardiovasc Surg. 1998. 116:353–354.

30. Rutten AP, Sikkenk PJ. Stapling devices in pulmonary surgery. Neth J Surg. 1982. 34:211–215.
31. Scott RN, Faraci RP, Goodman DG, Militano TC, Geelhoed GW, Chretien PB. The role of inflammation in bronchial stump healing. Ann Surg. 1975. 181:381–385.

32. Shields TW, Locicero IIIJ, Ponn RB, Rush VW. General Thoracic Surgery. 2004. 6th ed. Philadelphia: Williams & Wilkins;564.
33. Smiell J, Widmann WD. Bronchopleural fistulas after pneumonectomy. A problem with surgical stapling. Chest. 1987. 92:1056–1060.
34. Toloza EM, Harpole DH Jr. Intraoperative techniques to prevent air leaks. Chest Surg Clin N Am. 2002. 12:489–505.

35. Wertzel H, Wagner B, Hasse J, Lange W, Freudenberg N. Experimental gluing of the bronchial stump after pneumonectomy in rats. Eur J Cardiothorac Surg. 1997. 12:88–91.

36. Wright CD, Wain JC, Mathisen DJ, Grillo HC. Postpneumonectomy bronchopleural fistula after sutured bronchial closure: incidence, risk factors, and management. J Thorac Cardiovasc Surg. 1996. 112:1367–1371.

37. Yamamoto R, Inoue K, Hori T, Takehara S, Kaji M, Kinoshita H. Intercostal muscle pedicle flap for prophylaxis against bronchopleural fistula after pulmonary resection. Osaka City Med J. 1994. 40:99–105.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download