Abstract
We previously induced protective immune response by oral immunization with yeast expressing the ApxIIA antigen. The ApxI antigen is also an important factor in the protection against Actinobacillus pleuropneumoniae serotype 5 infection; therefore, the protective immunity in mice following oral immunization with Saccharomyces cerevisiae expressing either ApxIA (group C) or ApxIIA (group D) alone or both (group E) was compared with that in two control groups (group A and B). The immunogenicity of the rApxIA antigen derived from the yeast was confirmed by a high survival rate and an ApxIA-specific IgG antibody response (p < 0.01). The highest systemic (IgG) and local (IgA) humoral immune responses to ApxIA and ApxIIA were detected in group E after the third immunization (p < 0.05). The levels of IL-1β and IL-6 after challenge with an A. pleuropneumoniae field isolate did not change significantly in the vaccinated groups. The level of TNF-α increased in a time-dependent manner in group E but was not significantly different after the challenge. After the challenge, the mice in group E had a significantly lower infectious burden and a higher level of protection than the mice in the other groups (p < 0.05). The survival rate in each group was closely correlated to the immune response and histopathological observations in the lung following the challenge. These results suggested that immunity to the ApxIA antigen is required for optimal protection.
Most pathogens infect their host across mucosal surfaces, particularly those of the gastrointestinal tract or respiratory tract [24]. Immunoglobulin A (IgA) is the most abundant Ig isotype present in the mucosal tissue during infection and is crucial as a first line of defense. The main role of secretory IgA in oral immunization [8,22] is to protect the host by inhibiting pathogen attachment, immune exclusion, and facilitating the clearance of toxic products [37]. IgA may also function in lung defense by influencing the trafficking of specific cells through the common mucosal immune system [19]. The important roles that both specific local IgA and systemic IgG play in the protection from respiratory diseases have been well documented [11,12]. Although most bacterial extracts that are commonly administered orally produce nonspecific or poor immune responses, we previously demonstrated that the protection against Actinobacillus pleuropneumoniae increased with the production of specific IgA in the lung [34]. In addition, the induction of protective immunity in A. pleuropneumoniae infection by eliciting specific IgA and IgG after natural and experimental infection has been investigated [18].
A. pleuropneumoniae is the etiological agent of porcine pleuropneumonia, a severe respiratory disease affecting swine, is characterized by necrotizing fibrinous pneumonia and pleuritis [6]. Although the bacterium produces several virulence factors, the virulence of A. pleuropneumoniae is strongly correlated with the production of Apx exotoxins. Four different types of exotoxins, ApxI, ApxII, ApxIII and ApxIV, have been characterized in this bacterium [15,28]. Both ApxIA and ApxIIA of A. pleuropneumoniae are essential for full virulence in the development of clinical signs and typical lung lesions [5,28]. No preventive strategies have shown complete protection against the disease to date. Vaccination is thought to be the most effective way to prevent clinical signs by infection with the bacterium and many studies have focused on the development of novel vaccines to prevent A. pleuropneumoniae infection [5,17,18,26,32,39]. However, most vaccines have taken the form of injections, which are laborious and time-consuming, cause discomfort to the animal, and may cause adverse effects, such as the induction of an inflammatory response at the injection site [16,18,26].
Saccharomyces cerevisiae, commonly known as baker's yeast, has recently been adopted as a delivery vehicle for oral immunization [3]. This organism can express large quantities of heterogenous proteins at a relatively low cost [1,30] and is considered to be safe for human consumption [31]. In addition, S. cerevisiae has been used as a tracer for the oral application of vaccines and drugs because it is relatively stable, nonpathogenic, and noninvasive in the gut in comparison to other biodegradable vehicles [2,30]. The yeast may also stimulate the host mucosal immune system by interacting with intestinal epithelial cells in the presence of butyric acid, a metabolite produced by intestinal bacteria [29].
In addition to the induction of a specific antibody response, delivery systems and adjuvants are also key factors in designing an oral vaccine to efficiently induce a mucosal immune response [19,20,22]. Although several systems have been developed, they have failed to induce sufficient immune responses due to antigen dilution or denaturation, tight immune regulation at mucosal sites, toxicity, or insufficient immunostimulatory effects [27,40]. The recent success using S. cerevisiae as a delivery vehicle in oral immunization [3,4,29,38] led us to choose this yeast system for the delivery vehicle in our study.
Based on current knowledge, we propose that S. cerevisiae expressing Apx toxins is a more effective way to induce protective immunity against A. pleuropneumoniae infection than single administration of the ApxIIA. We first confirmed the immunogenicity of the yeast-derived ApxIA antigen. We then investigated the local and systemic immune responses, bacterial clearance, and inflammatory responses after oral immunization and challenge. Finally, we evaluated the protective efficacy of our vaccine strategy by challenge with a field isolate of A. pleuropneumoniae serotype 5.
Female 5-week-old BALB/c mice (Breeding and Research Center, Seoul National University, Korea) were used throughout this study in accordance with the policies and regulations for the care and use of laboratory animals (Seoul National University, Korea). All animals were provided with standard mouse chow and water ad libitum.
The immunogenicity of the ApxIA produced in the yeast was confirmed by subcutaneous immunization with yeast-derived ApxIA protein, and the survival rate after challenging with a clinical strain of A. pleuropneumoniae was determined as previously described [34].
Briefly, 15 mice per group were subcutaneously injected with 100 µg of protein extract after emulsifying with complete Freund's adjuvant (Sigma, USA). This was then followed by a boost immunization with the same amount of antigens after emulsifying with incomplete Freund's adjuvant (Sigma, USA) at 2 weeks after the initial immunization. The final immunization was performed in the same manner at 2 weeks after the boost immunization. Blood was drawn to collect serum at 5 days after the final boost immunization. Finally, a survival test and IgG antibody response assays were carried out in order to confirm the immunogenicity of the yeast-derived ApxIA antigen. Each experimental group in the oral vaccination study consisted of 40 mice, and each was allocated to one of five immunization regimens. Group A (control) received oral administration of 500 µl of 10 mM PBS (pH 7.2) and group B (vector) was orally vaccinated with 20 mg of S. cerevisiae powder dissolved into 500 µl of 10 mM PBS (pH 7.2). The vaccinated groups were immunized with 20 mg of S. cerevisiae expressing either ApxIA (group C), ApxIIA (group D), or both (10 mg each, group E) dissolved with the procedures as well.
All groups were immunized orally through an oral gavage with 4 doses at 10-day intervals. Five mice from each immunization group were randomly selected after 2 days (Fig. 1). Samples of lung, intestine, and serum were individually collected from the mice as described previously [34]. All serum samples were stored at -20℃ until use. Half of the lung and small intestine samples were homogenized with 10,000 RPM homogenization (Polytron PT3000; Kinematica, USA). The homogenized samples were stored at 4℃ overnight, then centrifuged at 12,000 ×g for 10 min at 4℃. The supernatants were collected for subsequent analysis and stored at -20℃ until use. The total protein concentration in each sample was measured using the BCA protein assay kit (Pierce, USA) and normalized to 1 mg immediately before performing the assay.
Antibody titers (IgA and IgG) against ApxIA or ApxIIA of A. pleuropneumoniae were measured by ELISA in order to analyze the immune response in the mice. For this assay, 100 µg of rApxIA and rApxIIA [33] resuspended in 100 µl of coating buffer (14.2 mM Na2CO3, 34.9 mM NaHCO3, 3.1 mM NaN3, pH 9.6) was added to a microplate for ELISA (Greiner, Australia) and incubated overnight at 4℃. The plate was washed three times with PBST (0.05% Tween 20 in PBS) and blocked with PBST containing 1% bovine serum albumin by incubation for 1 h at 37℃. After incubation with primary antigens, sera from the immunized mice, lung or intestinal homogenates, were added to the plate and incubated for 1 h at 37℃. After washing three times with PBST, 100 µl of goat anti-mouse IgG (H + L)-HRP conjugate (Bio-Rad, USA) or anti-mouse IgA (α-chain specific)-HRP conjugate (Sigma, USA) was added to the plate and incubated for 1 h at 37℃. Color was developed by adding 100 µl of ABTS substrate solution (Bio-Rad, USA) to the plate. After incubation for 20 min at room temperature, the O.D. was measured at 405 nm using an ELISA reader (Molecular Device, USA).
Immunohistochemical staining was followed by our previous report [34].
For tissue preparation, mice from each group were deeply anesthetized with a mixture of xylazine hydrochloride (Bayer, Korea) and ketamin hydrochloride (Yuhan, Korea) and then perfused intracardially with 0.9% saline, followed by a fixative (4% paraformaldehyde in 0.1 M PBS, pH 7.4) at a rate of 70 ml/min with a perfusion pump (Masterflex, USA). After perfusion, the lungs and intestines were removed and post-fixed overnight in the same fixative at 4℃. The lungs and intestines were cryoprotected by transfer to 30% sucrose in 0.1 M PBS and frozen in OCT embedding medium (Tissue-Tek; Sakura, USA) for storage at -70℃. Tissues were cut into 12 µm thick coronal sections with a cryostat (Reichert-Jung, Germany), mounted on silane-coated slides (DAKO, Denmark) and stored at -70℃ until processing for immunohistochemistry.
Tissue sections were rinsed with 0.01 M PBS (pH 7.4) and treated with 0.5% hydrogen peroxide in 0.01 M PBS for 15 min. The sections were washed three times for 10 min each with 0.01 M PBS, then blocked by incubation in 10% normal goat serum (DAKO, Denmark) or 10% skim milk in 0.1 M PBS for 1 h at room temperature. The sections were incubated with 50 µg/ml of rApxIA or rApxIIA in 0.1 M PBS overnight at 4℃. After incubation with primary antigens, the sections were washed three times with 0.01 M PBS for 10 min each and then incubated with 1 : 200 diluted polyclonal antibodies against a culture supernatant of A. pleuropneumoniae serotype 2 and 5 in 0.1 M PBS containing 0.3% triton X-100 and 2% normal goat serum for 2 h at room temperature. After washing with 0.01 M PBS for 10 min, the sections were sequentially reacted with 1 : 200 diluted goat anti-rabbit IgG (Vector, USA) and Streptavidin (Vector, USA) in the same solution. Between sequential reactions, the tissues were washed three times with PBS for 10 min each. The sections were visualized with 3'3-diaminobenzidine tetrachloride (Sigma, USA) in 0.1 M Tris buffer (pH 6.8) and mounted with a cover slide after counterstain with hematoxylin. Immunoreactive precipitates were observed under an Axioplan microscope (Carl Zeiss, Germany). Images of IgA immunoreactivity in ten villi in the small intestine and 10 alveolar spaces in the lung were randomly chosen from each animal and captured with an AppleScanner (Apple Computer, USA). The brightness and contrast of each image file were uniformly calibrated by Adobe Photoshop version 2.4.1, followed by analysis using NIH Image 1.59 software. Background staining values were subtracted from the immunoreaction intensities. The number of IgA-secreting cells in alveolar spaces was counted using Optimas 6.5 software (Media-Cybernetics, USA) by averaging the counts from 10 sections randomly taken from the same section level of each group.
Mice in each group were challenged by intraperitoneal injection of a field isolate of A. pleuropneumoniae serotype 5 at 1.45 × 106 CFU (minimal lethal dose, MLD) in 10 days after their final immunization, and were then monitored every 6 h for up to 72 h. During the monitoring, animals that succumbed to the challenge were dissected and lung tissues were collected for subsequent analysis of inflammatory responses, cytokines, and recovery.
To assess the protective efficacy measured by bacterial clearance in the lungs, lungs were aseptically removed at 72 h post-challenge. The lungs were homogenized in 5 ml of PBS using a tissue homogenizer. Each homogenate was serially diluted in PBS and 50 µl of the homogenate, and the diluted samples (in triplicate) were then plated on chocolate agar plates. The plates were incubated at 37℃ for 48 h under a 5% (V/V) CO2 atmosphere. The number of live bacteria was quantified according to the formula: CFU/ml = mean no. of colonies × dilution factor × 20. Differences were considered to be significant if a probability value of p < 0.05 was obtained when the CFU count of the immunized groups was compared to that of the control groups.
The mice were sacrificed at 72 h after challenge with the MLD of A. pleuropneumoniae serotype 5, and the lungs were sliced into pieces and preserved in 10% neutralized buffer formalin. The tissue samples were embedded in paraffin, cut into 6 µm sections, assessed by routine staining with hematoxylin and eosin, and examined by light microscopy. The inflammatory response was evaluated by examining the lung tissue for the presence of typical inflammatory signs [36]. Inflammatory index was obtained from the average of the score from each inflammatory response in 5 fields of each mouse. The severity of the inflammatory response (congestion, neutrophil infiltration, exudation, consolidation, infiltration of fibrosis and platelets) was ranked using a score of 0 to 3 for each symptom (0, no sign; 1, mild; 2, notable and local; 3, severe and spread) based on the size and number of lesions per field.
The levels of TNF-α, IL-1β, and IL-6 in the serum and lungs were quantified by ELISA (Endogen, USA) according to the instructions supplied by the manufacturer. Lung samples and sera from all experimental groups were prepared as described previously [9]. Briefly, aseptically prepared lungs were homogenized in 3 ml of lysis buffer. Lung homogenates were incubated on ice for 30 min and then centrifuged at 2,500 rpm for 10 min. The supernatants were collected and filtered using 0.45 µm syringe filters (Nalgen, USA). Before conducting the cytokine assessments, the protein concentration of each homogenate was normalized to 1 mg using a BCA protein assay kit (Pierce, USA). The amount of each cytokine was calculated by comparison with a standard curve generated by serial dilutions of murine recombinant cytokines.
Changes in IgA-secreting cells according to immunization time and treatment group were evaluated with ANOVA. The antibody titer and cytokine quantification results were expressed as the mean ± SD. Differences between control groups and vaccinated groups were analyzed by a two-tailed independent Student's t-test. Differences were considered to be significant if probability values of p < 0.05 were obtained.
To initially confirm the immunogenicity of the yeast-derived ApxIA antigen, the production of ApxIA-specific IgG antibodies and survival rates were investigated as in our previous study of the yeast-derived ApxIIA antigen [34]. The levels of ApxIA-specific IgG antibody were significantly increased by subcutaneous immunization with the protein extracted from the yeast expressing ApxIA. Mice challenged with the MLD of an A. pleuropneumoniae field isolate had a higher survival rate (70%) than the control (0%). None of the mice in the control groups showed significant production of specific antibody or protection against A. pleuropneumoniae after the challenge (data not shown).
The levels of local and systemic antibodies specific to the Apx antigens were investigated in mice orally immunized with Apx antigen-expressing yeast. The antibodies specific to ApxIA or ApxIIA were produced at similar levels in the group immunized with both the ApxIA and ApxIIA antigens. Mucosal immune responses were evaluated in the lung (Fig. 2A), intestine (Fig. 2B) and sera (Fig. 2C). Specific IgA responses to ApxIA or ApxIIA in the intestines and lungs from mice immunized with yeast expressing Apx antigens were significantly higher than those in the control groups after the second and third immunizations, respectively (p < 0.05). In particular, mice immunized with a single antigen (either ApxIA or ApxIIA) showed significant increases in the level of specific IgA at the final immunization (day 40) in both the lung and intestine (p < 0.05). However, no significant increases in specific IgA antibodies were observed in the sera of any experimental group, even though the levels of specific IgA were slightly higher in the vaccinated groups (p < 0.05) (Fig. 2C).
Systemically, the pattern of IgG production to ApxA antigens in the sera was similar to that of IgA. Increases in IgG antibodies were only observed in the group immunized with both antigens after the 2nd immunization and were maintained until the final immunization, while groups vaccinated with a single antigen showed no significant difference during the same period (p > 0.05) (Fig. 3A). Interestingly, similar levels of IgM antibody responses were observed in all vaccinated groups during the immunization period, while those in the two control groups remained unchanged (Fig. 3B).
The number of IgA-secreting cells in the lung and intestine was analyzed by counting the number of immunoreactive cells and densitometry. Representative specimens stained by immunohistochemistry for IgA-secreting cells in the lungs after the final immunization are shown in Fig. 4. The number of IgA-secreting cells significantly increased in the groups immunized with ApxIIA or both antigens after the third immunization, while the number of IgA-secreting cells in the group immunized with ApxIA increased only after challenge with A. pleuropneumoniae (Table 1). However, the relative densities of IgA-secreting cells in all vaccinated groups gradually increased after additional immunizations in comparison to the control groups. The final relative density of the groups immunized with ApxIA, ApxIIA, and both antigens were 8.5, 9.5 and 22.5 times higher than in the PBS-treated control group, respectively (Fig. 5).
The protective effect of oral immunization with yeast expressing ApxA antigens was also investigated through histopathological scoring and by measuring bacterial clearance at 72 h post challenge. Bacterial clearance was significantly enhanced by oral immunization with the antigens in all vaccinated groups (p<0.05) (Table 2). Moreover, the surviving mice showed significantly better clearance rates by 36 h post-challenge. The relationship between ApxA-specific antibody responses and bacterial counts from mouse lungs was further analyzed in the lung and sera from the control and vaccinated groups. Histopathological lesions, as measured by inflammatory indexes, were significantly reduced after vaccination while bacterial clearance rates were significantly increased. The lowest inflammatory index and the highest bacterial clearance rate were observed in the group immunized with both antigens (Table 2).
The levels of IL-6 and TNF-α significantly increased during immunization in the lungs from mice immunized with both antigens. However, the levels of IL-1β, IL-6 and TNF-α in the lungs of mice from the immunized groups did not change significantly after challenge, while the levels of these cytokines in the mice in the control groups significantly increased after challenge (Fig. 6). The cytokine levels in the sera were similarly raised only after challenge, with the exception of IL-1β, which did not change significantly (Fig. 6A). The production of TNF-α in both the sera and lung tissue of mice immunized with both antigens was slightly lower than that of the mice in the other groups after challenge.
All mice were monitored for up to 72 h after challenge with the MLD of an A. pleuropneumoniae field isolate. Overall, the final survival rates of the vaccinated groups were higher than those of the control groups at each time point. Notably, all mice in the control groups died at 36 h after challenge. The highest survival rate was observed in the group immunized with both antigens (Fig. 7).
The correlation coefficient (r2) was calculated by regression analysis in order to determine whether there was a correlation between survival rate and antibody response or the levels of bacterial colonization. The results showed that there was a statistically significant correlation (t test for correlation, p < 0.001) between the increase in mucosal IgA (r2 = 0.84), systemic IgG (r2 = 0.79), and survival rates. However, an increase in systemic IgA and IgM did not correlate with the survival rates. Moreover, the number of bacteria in the lung correlated negatively with the survival rate (r2 = 0.81).
Porcine pleuropneumonia caused by A. pleuropneumoniae is an important respiratory disease in the swine industry and has resulted in great economic loss worldwide [21]. Although the disease is multifactorial, vaccination has been considered to be the most effective strategy for protecting swine from A. pleuropneumoniae infection. Since most current vaccines are injected and may cause many adverse effects [17,18,26], alternative vaccines, including oral vaccines, have been sought after [8,18]. In addition, the induction of immune responses at remote mucosal effector sites through a common mucosal immune system has been demonstrated in animal models and has been partially confirmed in humans [12,13,22]. When developing an oral vaccine, it is essential to select an effective immunogen, appropriate adjuvant, and proper vaccine regimen [7,20]. We previously explored oral vaccination using yeast expressing the ApxIIA antigen as an alternative and convenient approach against A. pleuropneumoniae infection [34]. However, the protective effect of the oral immunization was not sufficient because the bacterium also produces other exotoxins. In this study, yeast expressing ApxIA were added as a vaccine component because ApxIA is also one of the most important factors associated with pathogenesis and protective immunity [17]. The efficacy of yeast expressing ApxIA or ApxIIA was evaluated using different vaccination regimens in a mouse model before being applied to the pigs. Mice immunized with proteins extracted from yeast expressing the ApxIA antigen produced strong IgG antibody responses and were protected against challenge, which suggests that the rApxIA antigen expressed in S. cerevisiae is highly immunogenic.
IgA and IgG immune responses increased following oral vaccination, and the highest level of response was observed in the group vaccinated with both S. cerevisiae that expressed ApxIA or ApxIIA. We also observed a large increase in antigen-specific IgA antibodies and the number of IgA-secreting cells in the intestine and lung. Based on the findings of other reports [7,8,34], these results strongly suggest that mucosal immune responses at remote sites induced by oral immunization are directly related to the effective production of IgA at the target mucosal site.
Only mice immunized with both ApxIA and ApxIIA produced sufficient humoral immune responses to Apx A toxins and consequently showed the highest survival against the challenge. These results compliment those of a previous report showing that exotoxins were required for the full virulence of A. pleuropneumoniae infection [5].
TNF-α and IL-6 production in the lung increased after vaccination, and IL-1β, TNF-α, and IL-6 production in the lung was abrogated only in the vaccinated groups after challenge with an A. pleuropneumoniae field isolate. This phenomenon might be due to the involvement of IL-6 in the production of IgA and the induction of TNF-α by IgA [23]. Moreover, the dual capacities of secreted IgA might be involved in the mechanism for maintaining balance between pro-inflammatory and anti-inflammatory activities [14,23]. In addition, the prevention of IL-1β, TNF-α and IL-6 production was correlated with a decrease in lung lesions in the vaccinated groups after challenge.
The highest bacterial clearance and survival rates were observed in the group immunized with both antigens. These results might indicate that oral vaccination using both antigens could induce more effective protection against particularly acute infections by decreasing mortality. It was also possible that IgA contributed to the protective mechanism by inhibiting the entrance of the pathogen into the lung and by modulating the pro-inflammatory responses [23,25]. The histopathological lesions, such as infiltration of inflammatory cells, were positively correlated with the groups showing high levels of inflammatory cytokine production. These results are in good agreement with those of previous studies in which inflammatory cell infiltration was mediated by inflammatory cytokines [9,10]. Although current thinking is that cell-mediated immunity does not play an important role in protection against A. pleuropneumoniae infection, the role of cell-mediated immune responses following oral immunization needs further investigation.
In conclusion, strains of S. cerevisiae that produce ApxA antigens could be a promising oral vaccine candidate for the prevention of A. pleuropneumoniae acute infection in pigs, alone or in combination with other bacterial components, and may provide optimal protection both systemically and at target mucosal sites.
Figures and Tables
Fig. 2
Specific-IgA antibody responses to Actinobacillus pleuropneumoniea AxpIIA or ApxIA toxin in the lung (A), small intestine (B), and sera (C) of mice orally immunized with S. cerevisiae (□, group A; ■, group B; ░, group C; ▧, group D; ▤, group E). Bars represent the mean O.D. values at 405 nm. Error bars represent the standard deviation from the mean. Significant differences between control groups and vaccinated groups are expressed as *p < 0.05 and **p < 0.01.

Fig. 3
Systemic specific IgG (A) and specific-IgM antibody responses (B) against Actinobacillus pleuropneumoniea AxpIIA or ApxIA toxin in the sera of mice orally immunized with S. cerevisiae (□, group A; ▪, group B; ░, group C; ▧, group D; ▤, group E). Bars represent the mean O.D. values at 405 nm. Error bars represent the standard deviation from the mean. Significant differences between the control and vaccinated groups are expressed as *p < 0.05 and **p < 0.01.

Fig. 4
Representative specimens stained by immunohistochemistry for IgA-secreting cells in the lungs of mice after the final immunization. A, group B; B, group D; and C, group E. Arrows indicate positive immunoreactive cells. Counterstaining with hematoxylin. ×400.

Fig. 5
Densitometric analysis of IgA immunoreactivity in the small intestines of mice orally immunized with S. cerevisiae (□, group A; ■, group B; ░, group C; ▧, group D; ▤, group E). Results are expressed as the mean relative density. Asterisks indicate significant differences from the PBS-treated group, *p < 0.05 and **p < 0.01.

Fig. 6
Comparison of pro-inflammatory cytokines IL-1β (A), IL-6 (B), and TNF-α (C) from the lung and sera of mice following oral immunization with S. cerevisiae (□, group A; ■, group B; ░, group C; ▧, group D; ▤, group E). Bars represent the mean concentration of cytokine proteins. Error bars represent the standard deviation from the mean.
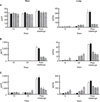
Fig. 7
Survival rates of mice immunized with S. cerevisiae after being challenged with the minimal lethal dose (MLD) of an A. pleuropneumoniae serotype 5 Korean isolate (-♦-, PBS-treated control; -□-, vector control; -▴-, oral immunization with 20 mg of S. cerevisiae expressing ApxIA antigen; -○-, oral immunization with 20 mg of S. cerevisiae expressing ApxIIA antigen; -*-, oral immunization with 10 mg each of S. cerevisiae expressing ApxIA and S. cerevisiae expressing ApxIIA antigen).

Table 1
Number of IgA-secreting cells in the lung following oral immunization in each experimental group

*Group A: PBS control. Group B: S. cerevisiae vector control. Group C: Oral vaccination with S. cerevisiae expressing ApxIA antigen. Group D: Oral vaccination with S. cerevisiae expressing ApxIIA antigen. Group E: Combined oral vaccination with S. cerevisiae-ApxIA and S. cerevisiae-ApxIIA antigen. Values are mean ± SD.
Table 2
Bacterial clearance in mice following oral immunization with yeast expressing rApxA antigens

*Each group is the same as Table 1.
Acknowledgments
This study was supported by BioGreen 21 (2005030134414), RDA, Brain Korea 21, and the Research Institute for Veterinary Sciences, Seoul National University, Korea.
References
1. Bathurst IC. Protein expression in yeast as an approach to production of recombinant malaria antigens. Am J Trop Med Hyg. 1994. 50:20–26.

2. Beier R, Gebert A. Kinetics of particle uptake in the domes of Peyer's patches. Am J Physiol. 1998. 275:G130–G137.

3. Blanquet S, Antonelli R, Laforet L, Denis S, Marol-Bonnin S, Alric M. Living recombinant Saccharomyces cerevisiae secreting proteins or peptides as a new drug delivery system in the gut. J Biotechnol. 2004. 110:37–49.

4. Blanquet S, Meunier JP, Minekus M, Marol-Bonnin S, Alric M. Recombinant Saccharomyces cerevisiae expressing P450 in artificial digestive systems: a model for biodetoxication in the human digestive environment. Appl Environ Microbiol. 2003. 69:2884–2892.

5. Boekema BK, Kamp EM, Smits MA, Smith HE, Stockhofe-Zurwieden N. Both ApxI and ApxII of Actinobacillus pleuropneumoniae serotype 1 are necessary for full virulence. Vet Microbiol. 2004. 100:17–23.

6. Bosse JT, Janson H, Sheehan BJ, Beddek AJ, Rycroft AN, Kroll JS, Langford PR. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect. 2002. 4:225–235.
7. Bouvet JP, Decroix N, Pamonsinlapatham P. Stimulation of local antibody production: parenteral or mucosal vaccination? Trends Immunol. 2002. 23:209–213.

8. Bowersock TL, Shalaby WS, Levy M, Samuels ML, Lallone R, White MR, Borie DL, Lehmeyer J, Park K. Evaluation of an orally administered vaccine, using hydrogels containing bacterial exotoxins of Pasteurella haemolytica, in cattle. Am J Vet Res. 1994. 55:502–509.
9. Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997. 65:1139–1146.

10. Choi C, Kwon D, Min K, Chae C. In-situ hybridization for the detection of inflammatory cytokines (IL-1, TNF-alpha and IL-6) in pigs naturally infected with Actinobacillus pleuropneumoniae. J Comp Pathol. 1999. 121:349–356.

11. Cox E, Van der Stedea Y, Verdonck F, Snoeck V, Van den Broeck W, Goddeeris B. Oral immunisation of pigs with fimbrial antigens of enterotoxigenic E. coli: an interesting model to study mucosal immune mechanisms. Vet Immunol Immunopathol. 2002. 87:287–290.

12. Dietrich G, Griot-Wenk M, Metcalfe IC, Lang AB, Viret JF. Experience with registered mucosal vaccines. Vaccine. 2003. 21:678–683.

13. Externest D, Meckelein B, Schmidt MA, Frey A. Correlations between antibody immune responses at different mucosal effector sites are controlled by antigen type and dosage. Infect Immun. 2000. 68:3830–3839.

14. Fernandez MI, Pedron T, Tournebize R, Olivo-Marin JC, Sansonetti PJ, Phalipon A. Anti-inflammatory role for intracellular dimeric immunoglobulin a by neutralization of lipopolysaccharide in epithelial cells. Immunity. 2003. 18:739–749.

15. Frey J. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 1995. 3:257–261.
16. Fuller TE, Martin S, Teel JF, Alaniz GR, Kennedy MJ, Lowery DE. Identification of Actinobacillus pleuropneumoniae virulence genes using signature-tagged mutagenesis in a swine infection model. Microb Pathog. 2000. 29:39–51.

17. Haga Y, Ogino S, Ohashi S, Ajito T, Hashimoto K, Sawada T. Protective efficacy of an affinity-purified hemolysin vaccine against experimental swine pleuropneumonia. J Vet Med Sci. 1997. 59:115–120.

18. Hensel A, van Leengoed LA, Szostak M, Windt H, Weissenbock H, Stockhofe-Zurwieden N, Katinger A, Stadler M, Ganter M, Bunka S, Pabst R, Lubitz W. Induction of protective immunity by aerosol or oral application of candidate vaccines in a dose-controlled pig aerosol infection model. J Biotechnol. 1996. 44:171–181.

19. Lauterslager TG, Hilgers LA. Efficacy of oral administration and oral intake of edible vaccines. Immunol Lett. 2002. 84:185–190.

20. Lauterslager TG, Stok W, Hilgers LA. Improvement of the systemic prime/oral boost strategy for systemic and local responses. Vaccine. 2003. 21:1391–1399.

21. Losinger WC. Economic impacts of reduced pork production associated with the diagnosis of Actinobacillus pleuropneumoniae on grower/finisher swine operations in the United States. Prev Vet Med. 2005. 68:181–193.

22. Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001. 14:430–445.

23. Olas K, Butterweck H, Teschner W, Schwarz HP, Reipert B. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol. 2005. 140:478–490.

24. Pabst R, Binns RM. The immune system of the respiratory tract in pigs. Vet Immunol Immunopathol. 1994. 43:151–156.

25. Pascual DW, Trunkle T, Sura J. Fimbriated Salmonella enterica serovar typhimurium abates initial inflammatory responses by macrophages. Infect Immun. 2002. 70:4273–4281.

26. Prideaux CT, Lenghaus C, Krywult J, Hodgson AL. Vaccination and protection of pigs against pleuropneumonia with a vaccine strain of Actinobacillus pleuropneumoniae produced by site-specific mutagenesis of the ApxII operon. Infect Immun. 1999. 67:1962–1966.

27. Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol Today. 1999. 20:493–500.

28. Reimer D, Frey J, Jansen R, Veit HP, Inzana TJ. Molecular investigation of the role of ApxI and ApxII in the virulence of Actinobacillus pleuropneumoniae serotype 5. Microb Pathog. 1995. 18:197–209.

29. Saegusa S, Totsuka M, Kaminogawa S, Hosoi T. Candida albicans and Saccharomyces cerevisiae induce interleukin-8 production from intestinal epithelial-like Caco-2 cells in the presence of butyric acid. FEMS Immunol Med Microbiol. 2004. 41:227–235.

30. Schreuder MP, Deen C, Boersma WJ, Pouwels PH, Klis FM. Yeast expressing hepatitis B virus surface antigen determinants on its surface: implications for a possible oral vaccine. Vaccine. 1996. 14:383–388.

31. Schreuder MP, Mooren AT, Toschka HY, Verrips CT, Klis FM. Immobilizing proteins on the surface of yeast cells. Trends Biotechnol. 1996. 14:115–120.

32. Seah JN, Frey J, Kwang J. The N-terminal domain of RTX toxin ApxI of Actinobacillus pleuropneumoniae elicits protective immunity in mice. Infect Immun. 2002. 70:6464–6467.

33. Shin SJ, Cho YW, Yoo HS. Cloning, sequencing and expression of apxIA, IIA, IIIA of Actinobacillus pleuropneumoniae isolated in Korea. Korean J Vet Res. 2003. 43:247–253.
34. Shin SJ, Bae JL, Cho YW, Lee DY, Kim DH, Yang MS, Jang YS, Yoo HS. Induction of antigen-specific immune responses by oral vaccination with Saccharomyces cerevisiae expressing Actinobacillus pleuropneumoniae ApxIIA. FEMS Immunol Med Microbiol. 2005. 43:155–164.

35. Shin SJ, Bae JL, Cho YW, Yang MS, Kim DH, Jang YS, Yoo HS. Expression of apxIA of Actinobacillus pleuropneumoniae in Saccharomyces cerevisiae. J Vet Sci. 2003. 4:225–228.

36. Shin SJ, Wu CW, Steinberg H, Talaat AM. Identification of novel virulence determinants in Mycobacterium paratuberculosis by screening a library of insertional mutants. Infect Immun. 2006. 74:3825–3833.

37. Silin DS, Lyubomska V. Overcoming immune tolerance during oral vaccination against Actinobacillus pleuropneumoniae. J Vet Med B Infect Dis Vet Public Health. 2002. 49:169–175.

38. Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, Franzusoff A, Duke RC, Wilson CC. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001. 7:625–629.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download