Abstract
This study compared the effects of high linear energy transfer (LET) fast neutrons on the induction of apoptosis in the hair follicles of ICR mice with those of low LET 60Co γ-rays. The changes that occurred from 0 to 24 h after exposing the mice to either 2 Gy of γ-rays (2 Gy/min) or 0.8 Gy of neutrons (94 mGy/min, 35 MeV) were examined. The maximum frequency was found at 12 h (γ-rays) or 8 h (neutrons) after irradiation. The mice that received 0-8 Gy of γ-rays or 0-1.6 Gy of neutrons were examined 8 h after irradiation. The dose-response curves were analyzed using the best-fit curve model. The dose-response curves were linear-quadratic, and a significant relationship was found between the frequency of apoptotic cells and the dose. The morphological findings in the irradiated groups were typical apoptotic fragments in the matrix region of the hair follicle, but the spontaneous existence of apoptotic fragments was rarely observed in the control group. In the presence of an apoptosis frequency between 2 and 14 per follicle, the relative biological effectiveness values of neutrons in small and large follicles were 2.09 ± 0.30 and 2.15 ± 0.18, respectively.
The hair follicle and its hair have long been recognized as potentially useful biological indicators for the quantitative index of radiation injury in nuclear and medical radiation. Hairs are located over much of the body surface and can provide regional information. Therefore, the skin and its appendages would appear to offer the only system in which the dose distribution of radiation over the body surface may be assessed by an estimate of the received dose on a suitable time-scale for clinical intervention [5,12,31].
Apoptosis is a spontaneous or induced phenomenon that can be observed in many cell types [17]. Radiation-induced programmed cell death is a degradative and progressive process. The degradative process is initiated in the target nucleus, ultimately resulting in the quantitative conversion of the target genome into small DNA fragments. Apoptosis is initiated not only by pathological conditions, but is also triggered by factors such as cellular mechanisms intrinsically or extrinsically regulated by physiological stimuli. Radiation-induced apoptosis has mainly been characterized in lymphocytes in vitro, and appears to be related to the number of DNA strand breaks, the rate at which they occur, and the rapidity and effectiveness of the DNA repair mechanisms [2,9,22]. However, other results suggest that DNA might not be the only target that induces an apoptotic stimulus after irradiation that could mainly involve cell membrane damage [20,27]. These cellular studies do not take into account cell-to-cell interactions and cell differentiation processes that can play important roles during the initiation and progression of apoptosis. These roles can be examined using histological methods, and the few available data have mostly come from extremely radiation-sensitive tissues such as the adult gut [25] or the central nervous system during histogenesis [7,16,18].
The biological effects of fast neutrons in normal tissues and in tumors are of interest in relation to clinical radiotherapy, for radiation protection purposes, and to aid in the basic understanding of the radiation-induced inactivation of cells, whether by low or high linear energy transfer (LET) radiation. In general, the biological effects of high-LET radiation are greater than those of low-LET radiation. The variations in the relative biological effectiveness (RBE) with dose, with oxygenation, with cell cycle parameters, and from one tissue to another are well-documented [3]. However, few data are available on apoptosis in hair follicles exposed to radiation at higher ionizing density, such as neutrons. In this study, we used cyclotron-derived fast neutrons with a peak energy of 35 MeV to investigate how the energy of neutrons affects the biological processes. We evaluated the RBE for fast neutron-induced apoptosis in the hair follicles using ICR mice compared with the results of parallel experiments using γ-rays.
Male ICR mice were obtained from a specific pathogen-free colony (Oriental Bio, Korea), and were allowed 1 week of quarantine and acclimatization. The animals were housed in a room that was maintained at 23 ± 2℃, with a relative humidity of 50 ± 5%, artificial lighting from 08 : 00 to 20 : 00, and 13-18 air changes per hour. The animals were housed four per stainless steel wire mesh cage, and were given tap water and commercial rodent chow (Samyang Feed, Korea) ad libitum. ICR mice between the ages of 7 and 8 weeks were used. At this age, the skin of the mice contains a synchronous resting population of hair follicles (telogen phase). These resting follicles can be stimulated into activity by the simple act of plucking a liquid plastic dressing (Alteco-Ace; Alteco Korea, Korea), which dries within 10 min after application, and can be removed from the animals together with the embedded hairs. Ten days after plucking, the follicles were in mid-anagen and the animals were subjected to whole-body irradiation with either γ-rays or fast neutrons. The neutrons were generated from the KCCH cyclotron using the proton-beryllium reaction. The estimated forward neutron spectra established a peak energy of 35 MeV. The mean dose rate for neutrons was 94 mGy/min. The contamination of γ-rays was estimated as 14.2% of the neutron dose. Exposure to 137Cs-generated γ-rays was conducted with Gammacell (Nordion, Canada). The mean dose rate of γ-rays was 2 Gy/min. Fifty-two mice were assigned to thirteen groups: non-irradiated control, fast neutron (0.8 Gy), and γ-rays (2 Gy). The mice were sacrificed at various periods from 2 to 24 h after irradiation (four mice for each time interval). Forty mice were exposed to 0, 0.2, 0.4, 0.8, or 1.6 Gy of neutrons or 0.5, 1.0, 2.0, 4.0, or 8.0 Gy of γ-rays, and were sacrificed at 8 h after irradiation. The Institutional Animal Care and Use Committee at Chonnam National University approved the protocols used in this study, and the animals were cared for in accordance with the Guidelines for Animal Experiments.
Skin samples obtained from the mid-dorsum were fixed with 10% neutral-buffered formalin. The skin was first flattened onto a piece of paper to prevent it from curling during fixation. Three micrometer sections were cut on a plane parallel to the long axis of the animal rather than across the animal. These provided longitudinal sections of the follicles that were aligned parallel to the long axis. In order to visualize the apoptotic cells, we used the TdT-mediated dUTP-biotin nick end-labeling (TUNEL) method of immunohistochemical staining with a commercial kit (ApopTag Plus Peroxidase In Situ Apoptosis Detection kit; Intergen, USA), and stained the cells with hematoxylin and eosin (H&E).
The follicles were selected for scoring of apoptotic cells if they were good examples of longitudinal sections. In practice, this meant that they had to contain the developing hair root and a full longitudinal section of the dermal papilla. They were subjectively grouped into large or small follicles, and 20 of each were scored using an oil immersion (×1,000). Large follicles are most likely to be those responsible for the three types of guard hairs, while small follicles are responsible for the small underfur or zigzag hairs. All analyses were performed using the Graph PAD In Plot computer program (Graph Pad Software, USA).
The apoptotic cells, which are primarily found in the matrix region of the follicle, were easily recognized from the condensation of their cytoplasm and nuclear chromatin. The dead cells break up into several fragments. Not all of the fragments necessarily contain fragments of the cell nucleus. These cytoplasmic fragments can usually be recognized by their eosinophilic staining properties in H&E stain. Apoptosis was easily recognized by the presence of whole apoptotic bodies showing peroxidase staining. In the TUNEL-positive cells or bodies, the stained products exactly correlated with the typical morphological characteristics of apoptosis as seen at the light microscopic level (Fig. 1). A small number of cells in the hair follicle exhibited apoptosis in the sham-irradiated mice at the levels of 0.10 (small) and 0.21 (large) per follicle. At 2 h after irradiation, there was an increase in the number of apoptotic cells, and the maximal frequency was found at 12 h (γ-ray) or 8 h (neutron) after irradiation (Fig. 2).
Table 1 shows the amount of cell death caused by apoptosis at each dose. Apoptotic cell death, which was occasionally found in the control animals, was markedly enhanced by irradiation. The dose-response curves were analyzed using the best-fit curve model. The dose-response curves were linear-quadratic, and a significant relationship was found between the frequency of apoptotic cells and the dose (Fig. 3). Taking the controls into account, the lines of best-fit are as follows:
γ-rays:
small follicles: y = (3.573 ± 0.0356)D + (-0.222 ± 0.00498) D2 + (0.114 ± 0.0085), γ2 = 1.0
large follicles: y = (6.000 ± 0.2755)D + (-0.372 ± 0.03848)D2 + (0.210 ± 0.0115), γ2 = 0.995 ;
Neutrons:
small follicles: y = (6.034 ± 0.5289)D + (-0.342 ± 0.36884) D2 + (0.114 ± 0.0085), γ2 = 0.995
large follicles: y = (10.979 ± 1.619)D + (-0.00935 ± 1.1291) D2 + (0.210 ± 0.0115), γ2 = 0.989;
where y is the number of apoptotic cells per follicle and D is the irradiation dose in Gy.
Since the neutrons cause mixed neutron-γ radiation, the rate of induction by neutrons (Vn+γ) can be approximated by Vn+γ = pVn + (1 - p)Vγ, where p is the fraction of the neutron dose contributing to the total dose of fast neutrons, Vn is the value induced by neutrons, and Vγ is the value induced by γ-rays. Vn + γ = pVn + (1 - p)Vγ can be rewritten as Vn = Vγ + (Vn+γ - Vγ) ÷ p. When analyzed by the linear-quadratic model, the lines of best-fit of the theoretical dose-response to neutrons are as follows:
small follicles: y = (6.44135 ± 0.6164)D + (-0.3627 ± 0.4299) D2+ (0.114 ± 0.0085), γ2 = 0.994;
large follicles: y = (11.5469 ± 1.464)D + (0.216648 ± 2.099) D2 + (0.210 ± 0.0115), γ2 = 0.985.
In order to determine the RBE of neutrons compared with γ-rays, the equation, y = aD + bD2 + c was transformed as D = [-a ± √ {a2 - 4b(c - y)}] ÷ 2b. The RBEs of the neutrons were obtained from this equation. In the presence of an apoptosis frequency between 2 and 14 per follicle, the RBEs of the neutrons in the small and large follicles were 2.09 ± 0.30 and 2.15 ± 0.18, respectively (Table 2).
The recognition that apoptotic cell death can be a major component of radiation damage, particularly in rapidly proliferating cell populations, has important implications in radiobiological studies. Since hair loss following exposure to radiation is a well-recognized phenomenon, the hair follicle has been shown to be a radiosensitive organ. However, there have been relatively few studies of the parameters related to the dose-response relationships for radiation-induced damage [19,24,30].
The data obtained in this study indicate that there is a quantitative change in apoptotic cells that are produced by various doses of radiation. The morphological findings for the apoptotic cells showed chromatin condensation into the crescent caps at the nuclear periphery, along with nuclear disintegration, a decrease in nuclear size, a reduction of the cell volume, and an increase in cell density in the hair follicles. The location of the target cells against radiation-induced programmed cell death in the hair follicles has not been adequately elucidated in previous studies, but data has shown that most of the cells that are killed by apoptosis are found in the lower regions of the follicle germinal matrix. These findings indicate that some of the follicle stem cells are sensitive to this programmed cell death. The quantification of the apoptotic fragments is a more sensitive and accurate assay than the other scoring systems based on visual observations [19,26,30].
The hairs and their follicles are readily accessible, easily sampled, and cover most of the skin surface. As such, they represent the only system that can be used to estimate the local doses or dose distributions of radiation over the body surface. Therefore, an examination of the whole follicle would be a more sensitive means of detecting radiation damage than other biological indicators, particularly because radiation-induced cell damage in the growing hair matrix can usually be detected within a few hours in sectioned follicles. This effect is similar in appearance to that observed in crypt cells of the small intestine. This index is known to be one of the most sensitive radiobiological endpoints [14,15].
The present study was the first to show a dose-response relationship for neutron-induced apoptosis in hair follicles. The dose-response curve for the neutron-irradiation groups was much steeper than that for the γ-irradiated groups. The yield of the cells undergoing apoptosis appears to show a linear-quadratic relationship to the dose. It is generally known that the dose-effect relationship in the cell death induced by neutrons is best fit to a linear model, while low-LET radiation-induced cell death fits a linear-quadratic model. However, most of these data have been derived from in vitro studies with acute high dose irradiation. Several in vivo experiments that demonstrated the dose-response curves of some neutron-induced tissue injuries were fir to the linear-quadratic model, such as the normal tissues reviewed by Broerse and Barendsen [4] and IARC [13].
Although a wide range of RBE values has been reported for fast neutrons [29], an RBE value near 1 was reported for radiation-induced apoptosis in human lymphocytes exposed to high-energy 14.5 MeV neutrons [33]. Due to the spread in the measured RBE values in various tissues, it is still difficult to estimate the RBE associated with this radiation quality, which indicates the need for further research to resolve this issue. Apoptosis is the most sensitive indicator of the radiation response. Hendry et al. [10,11] calculated an RBE of 4 for the apoptosis data in the mouse small intestine irradiated with fast neutrons with an energy of 14.7 MeV. Warenius et al. [34] reported an RBE of 1.0 for the apoptosis data of mouse thymocytes irradiated with fast neutrons at 62.5 MeV. Here, we showed that the RBEs of fast neutrons were 2.09 (small follicle) and 2.15 (large follicle) in the presence of an apoptosis frequency between 2 and 14 per follicle. Therefore, it appears that the RBE for apoptosis is tissue-dependent. On the other hand, Fujikawa et al. [8] calculated an RBE of 4.6 for the apoptosis of thymocytes in mice irradiated with fission neutrons. Therefore, the small RBE value of thymocyte apoptosis reported by Warenius et al. [34] could be ascribed to the large energy of neutrons.
The RBE estimated for fast neutrons in this study was greater than unity. This means that the apoptosis assay in mouse hair follicles is sensitive to a difference in radiation quality. The reported studies of DNA damage induced by radiation of different qualities have generally shown a relatively higher fraction of non-rejoining DNA double-strand breaks (DSBs) after high-LET radiation [1,6,28,32]. In addition, high-LET radiations and gamma-rays have been shown to produce initial DSBs, although they are of different quality, with similar efficiency in cultured rodent cells [21,23]. Overall, it is believed that DSB repair in the hair follicle is involved as a determinant of the RBE of high-LET radiation for induced apoptotic cell formation in hair follicles.
In summary, this study determined the time-response relationships of apoptotic cell formation in the hair follicles of ICR mice for fast neutrons and γ-rays, and established a linear-quadratic dose-effect relationship for both types of radiation. Based on the dose-response data, the RBE values of fast neutrons were estimated to be 2.09 for small follicles, and 2.15 for large follicles. Further mechanistic studies on the effects of neutron-induced apoptosis in the hair follicle will be needed to extrapolate the experimental data for protection against radiation in humans.
Figures and Tables
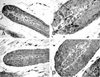 | Fig. 1Photomicrograph of small (A, C) and large (B, D) hair follicles of mice sacrificed 8 h after irradiation. The apoptotic cells, which occur predominantly in the matrix region of the follicle, were easily recognized from the condensation of their cytoplasm and nuclear chromatin. A and B; H&E staining, C and D; TUNEL staining, ×400. |
 | Fig. 2Variation in apoptotic cell frequency in small (□) or large (▪) hair follicle with time after whole-body irradiation of ICR mice with 0.8 Gy of fast neutrons (A) and 2.0 Gy of γ-rays (B). Results are presented as means ± SD from four mice in each group. |
 | Fig. 3Dose-response for fast neutrons (▪) and γ-rays (•) induced apoptotic cells in small (A) or large (B) hair follicle. The lines represent the results of a linear-quadratic fit through the data indicated in the figure. |
Acknowledgments
This work was supported by a Korea Science and Engineering Foundation (KOSEF) Grant funded by the Government (MOST), Korea.
References
1. Blöcher D. DNA double-strand break repair determines the RBE of α-particles. Int J Radiat Biol. 1988. 54:761–771.

2. Bonicalzi ME, Haince JF, Droit A, Poirier GG. Regulation of poly(ADP-ribose) metabolism by poly(ADP-ribose) glycohydrolase: where and when? Cell Mol Life Sci. 2005. 62:739–750.
3. Britten RA, Peters LJ, Murray D. Biological factors influencing the RBE of neutrons: implications for their past, present and future use in radiotherapy. Radiat Res. 2001. 156:125–135.

4. Broerse JJ, Barendsen GW. Relative biological effectiveness of fast neutrons for effects on normal Tissues. Curr Top Radiat Res Q. 1973. 8:305–350.
5. Denekamp J, Joiner MC, Maughan RL. Neutron RBEs for mouse skin at low doses per fraction. Radiat Res. 1984. 98:317–331.

6. Fox JC, McNally NJ. The rejoining of DNA double-strand break following irradiation with 238Pu α-particles: evidence for a fast component of repair as measured by neutral filter elution. Int J Radiat Biol. 1990. 57:513–521.

7. Fritsch P, Richard-Le Naour H, Denis S, Ménétrier F. Kinetics of radiation-induced apoptosis in the cerebellum of 14-day-old rats after acute or during continuous exposure. Int J Radiat Biol. 1994. 66:111–117.

8. Fujikawa K, Hasegawa Y, Matsuzawa S, Fukunaga A, Itoh T, Kondo S. Dose and dose-rate effects of X rays and fission neutrons on lymphocyte apoptosis in p53(+/+) and p53(-/-) mice. J Radiat Res (Tokyo). 2000. 41:113–127.

9. Hale AJ, Smith CA, Sutherland LC, Stoneman VE, Longthorne VL, Culhane AC, Williams GT. Apoptosis: molecular regulation of cell death. Eur J Biochem. 1996. 236:1–26.

10. Hendry JH, Potten CS, Chadwick C, Bianchi M. Cell death (apoptosis) in the mouse small intestine after low doses: effects of dose-rate, 14.7 MeV neutrons, and 600 MeV (maximum energy) neutrons. Int J Radiat Biol Relat Stud Phys Chem Med. 1982. 42:611–620.

11. Hendry JH, Potten CS, Merritt A. Apoptosis induced by high- and low-LET radiations. Radiat Environ Biophys. 1995. 34:59–62.

12. Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990. 57:751–773.

13. IARC. Neutrons. IARC Monogr Eval Carcinog Risks Hum. 2000. 75 Pt 1:363–448.
14. Ijiri K, Potten CS. Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br J Cancer. 1983. 47:175–185.

15. Ijiri K, Potten CS. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer. 1987. 55:113–123.

16. Ishida Y, Ohmachi Y, Nakata Y, Hiraoka T, Hamano T, Fushiki S, Ogiu T. Dose-response and large relative biological effectiveness of fast neutrons with regard to mouse fetal cerebral neuron apoptosis. J Radiat Res. 2006. 47:41–47.

17. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972. 26:239–257.

18. Kim SH, Chung CY, Son CH. Cell death by apoptosis in the neonatal mouse cerebellum following gamma-irradiation. Anticancer Res. 1998. 18:1629–1632.
19. Kim SH, Kim SR, Lee HJ, Oh H, Ryu SY, Lee YS, Kim TH, Jo SK. Apoptosis in growing hair follicles following gamma-irradiation and application for the evaluation of radioprotective agents. In Vivo. 2003. 17:211–214.
21. Kysela BP, Arrand JE, Michael BD. Relative contribution of levels of initial damage and repair of double-strand breaks to the ionizing radiation-sensitive phenotype of the Chinese hamster cell mutant, XR-V15B. Part II. Neutrons. Int J Radiat Biol. 1993. 64:531–538.

22. Maity A, McKenna WG, Muschel RJ. The molecular basis for cell cycle delays following ionizing radiation: a review. Radiother Oncol. 1994. 31:1–13.

23. Newman HC, Prise KM, Folkard M, Michael BD. DNA double-strand break distributions in X-ray and α-particle irradiated V79 cells: evidence for non-random breakage. Int J Radiat Biol. 1997. 71:347–363.
24. Potten CS. Biological dosimetry of local radiation accidents of skin: possible cytological and biochemical methods. Br J Radiol Suppl. 1986. 19:82–85.
25. Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol. 1990. 58:925–973.

26. Potten CS, Geng L, Taylor P. Hair medullary cell counts: a simple and sensitive indicator of radiation exposure. Int J Radiat Biol. 1990. 57:13–21.

27. Ramakrishnan N, McClain DE, Catravas GN. Membranes as sensitive targets in thymocyte apoptosis. Int J Radiat Biol. 1993. 63:693–701.

28. Ritter MA, Cleaver JE, Tobias CA. High-LET radiations induce a large proportion of non-rejoining DNA breaks. Nature. 1977. 266:653–655.

29. Ryan LA, Wilkins RC, McFarlane NM, Sung MM, McNamee JP, Boreham DR. Relative biological effectiveness of 280 keV neutrons for apoptosis in human lymphocytes. Health Phys. 2006. 91:68–75.

30. Sieber VK, Sugden EM, Alcock CJ, Belton RR. Reduction in the diameter of human hairs following irradiation. Br J Radiol. 1992. 65:148–151.

31. Song S, Lambert PF. Different responses of epidermal and hair follicular cells to radiation correlate with distinct patterns of p53 and p21 induction. Am J Pathol. 1999. 155:1121–1127.

32. Van der Sachans GP, Paterson MC, Cross WG. DNA strand break and rejoining in cultured human fibroblasts exposed to fast neutrons or gamma-rays. Int J Radiat Biol Relat Stud Phys Chem Med. 1983. 44:75–85.
33. Vral A, Cornelissen M, Thierens H, Louagie H, Philippé J, Strijckmans K, De Ridder L. Apoptosis induced by fast neutrons versus 60Co gamma-rays in human peripheral blood lymphocytes. Int J Radiat Biol. 1998. 73:289–295.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download