Abstract
The purpose of this study was to determine the expression and distribution of band 3 in the collecting duct and connecting tubules of the kidney of the marmoset monkey (Callithrix jacchus), and to establish whether band 3 is expressed in type A intercalated cells. The intracellular localization of band 3 in the different populations of intercalated cells was determined by double-labeling immunohistochemistry. Immunohistochemical microscopy demonstrated that band 3 is located in the basolateral plasma membranes of all type A intercalated cells in the connecting tubule (CNT), cortical collecting duct (CCD), and outer medullary collecting duct (OMCD) of the marmoset. However, type B intercalated cells and non-A/non-B intercalated cells did not show band 3 labeling. Electron microscopy of the CNT, CCD and OMCD confirmed the light microscopic observation of the basolateral plasma membrane staining for band 3 in a subpopulation of interacted cells. Basolateral staining was seen on the plasma membrane and small coated vesicles in the perinuclear structure, some of which were located in the Golgi region. In addition, there was no labeling of band 3 in the mitochondria of the CNT, CCD and in OMCD cells. The intensity of the immunostaining of the basolateral membrane was less in the CNT than in the CCD and OMCD. In contrast, band 3 immunoreactivity was greater in the intracellular vesicles of the CNT. From these results, we suggest that the basolateral Cl-/HCO3- exchanger in the monkey kidney is in a more active state in the collecting duct than in the CNT.
The collecting duct of the mammalian kidney plays a major role in urine acidification. The mammalian collecting duct is composed of two structurally and functionally distinct cell types, principal cells and intercalated cells [10, 16]. Although intercalated cells are considered as characteristic of the collecting duct, they are also present in the connecting segment or connecting tubule (CNT) [5]. Intercalated cells constitute approximately one-third of the cells in the CNT, as well as in the cortical collecting duct (CCD) and outer medullary collecting duct (OMCD) [10]. Based on the apical/basolateral locations of the Cl-/HCO3- exchanger and H+-ATPase, the present classification distinguishes three types of intercalated cells: type A (basolateral Cl-/HCO3- exchanger and apical H+-ATPase), type B (apical Cl-/HCO3- exchanger and basolateral H+-ATPase), and non-A/non-B (apical Cl-/HCO3- exchanger and H+-ATPase) [13]. Electrophysiological studies, mostly conducted on rabbit CCDs and CNTs, have defined the conductance properties of type A and type B intercalated cells using the in vitro microperfusion technique and membrane potential recordings with microelectrodes [4,12]. Band 3 (AE1 or anion exchange protein 1, the product of the CDB3 gene) was first identified and characterized in the human erythrocyte. Subsequent studies have revealed that band 3 or one of its anion exchanger homologues (AE2 and AE3) is found in most cells of the body [7,8]. In the human, rat, mouse, and rabbit kidneys [6], band 3 (AE1) is localized primarily to the basolateral membrane of a subset of intercalated cells of the CCD and MCD. At this location, band 3 is thought to facilitate the exchange of Cl- for HCO3-, a process involved in pH regulation by the kidney [19]. Other studies have established that type A and type B cells are present in the CCD and the CNT of rats [2,18], mice [15], and rabbits [14]. However, there has been no report of the localization pattern of band 3 in the kidney of the common marmoset monkey.
The aim of this study was to establish the localization pattern of band 3 in intercalated cells in the kidney of the common marmoset monkey. Specific antibodies directed against H+-ATPase and band 3 proteins were used to identify subpopulations of intercalated cells.
In this study, we used three male common marmoset monkeys (Callithrix jacchus), ranging in age from two to three years, obtained from the Genetic Resources Center at the Korea Research Institute of Bioscience and Biotechnology. The animals were housed at a constant temperature (23℃) and relative humidity (60%) with a fixed 12 h light/dark cycle and free access to food and water. Procedures involving animals and their care conformed to the institutional guidelines, which comply with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, USA).
Animals were anesthetized with sodium pentobarbital injections (40 mg/kg body weight, i.p.; Abbott, USA) and were perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) containing 0.1% sodium nitrite and 1 U/100 ml of heparin, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). After perfusion, the kidneys were excised and cut into slices, which were fixed by immersion in the same fixative solution for 2 h at 4℃. Sections of tissue were cut transversely through the entire kidney with a vibratome at a thickness of 50 µm and were processed for immunohistochemical studies using a horseradish-peroxidase preembedding technique.
Band 3 immunoreactivity was detected with an affinity-purified rabbit polyclonal antibody directed against human erythrocyte band 3 protein. The antibody has been characterized in a previous study [8]. An H+-ATPase-directed antibody was used to identify intercalated cells. This antibody is capable of labeling all intercalated cell subtypes in both the mouse and rat [5].
The vibratome sections (50 µm) were processed for immunohistochemistry using an indirect preembedding immunoperoxidase method. All sections were washed three times for 15 min each with 50 mM NH4Cl in PBS. Before incubation with the primary antibody, the sections were pretreated for 3 h with PBS containing 1% bovine serum albumin (BSA), 0.05% saponin, and 0.2% gelatin (solution A). The sections were then incubated overnight at 4℃ with antibodies directed against band 3 diluted 1 : 100,000 in 1% BSA in PBS (solution B). Control incubations were performed in solution B lacking the primary antibody. After three washes with solution A, the sections were incubated for 2 h with a peroxidase-conjugated donkey anti-goat or anti-rabbit IgG Fab fragment (Jackson, USA) diluted 1 : 100 in solution B. The tissues were rinsed first in solution A and were then rinsed in 0.05 M Tris buffer (pH 7.6). For the detection of horseradish peroxidase, the sections were incubated in 0.1% 3,3'-diaminobenzidine (DAB) in 0.05 M Tris buffer (pH 7.6) for 5 min, after which H2O2 was added to a final concentration of 0.01%, and the incubation was continued for 10 min. After the sections had been washed with 0.05 M Tris buffer (pH 7.6), they were dehydrated in a graded series of ethanol. The vibratome sections (50 µm thick) through entire kidneys from all the animals were embedded in Epon 812, and were sandwiched between polyethylene vinyl sheets.
Each region was excised from the flat-embedded vibratome sections of kidneys processed for the immunohistochemical identification of type A intercalated cells using band 3 protein, and was glued onto an empty block of Epon-812. Two consecutive 1 µm sections were cut for double immunolabeling for H+-ATPase. The sections were treated for 15 min with a mixture of saturated sodium hydroxide and absolute ethanol (1 : 1) to remove the resin. After three brief rinses in absolute ethanol, the sections were hydrated with graded ethanol and were rinsed in tap water. The sections were then rinsed with PBS, incubated in normal donkey serum for 1 h, and were then incubated overnight at 4℃ with an antibody directed against H+-ATPase. After the sections had been washed in PBS, the sections were incubated for 2 h in peroxidase-conjugated donkey anti-goat or anti-rabbit IgG (Fab fragment), and were washed again with PBS. In detecting H+-ATPase, Vector SG (Vector, USA) was used as the chromogen to produce a gray-blue color, which is easily distinguishable from the brown label produced by DAB in the first immunolocalization procedure for band 3, using the preembedding method. The sections were washed with distilled water, dehydrated with graded ethanol and xylene, mounted in balsam, and examined by light microscopy.
Electron microscopic observations were made of the vibratome sections postfixed with 1% glutaraldehyde and 1% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4) before they were dehydrated and embedded in poly/Bed 812 resin. Ultrathin sections were stained with uranyl acetate and lead citrate, and photographed using a transmission electron microscope (1200EX; JEOL, Japan).
To evaluate the overall distribution of band 3 in marmoset monkey kidney, immunohistochemical analysis was performed with preembedding immunostaining of 50 µm thick vibratome sections. Immunohistochemical staining revealed abundant labeling of the CNT and CCD in the cortex and the OMCD (Fig. 1).
To establish that band 3 is expressed in type A intercalated cells, we used a double-labeling immunohistochemical procedure for H+-ATPase and band 3 protein on 1 µm thick sections from the marmoset kidney. Type A intercalated cells, identified by the presence of apical H+-ATPase and basolateral band 3 immunostaining, constituted a major portion of the intercalated cells in the CNT, CCD, and OMCD of the marmoset kidney. Type B intercalated cells secrete HCO3- via an apical Cl-/HCO3- exchanger that is functionally distinct from the basolateral apical Cl-/HCO3- exchanger in the type A intercalated cells. Thus, type B intercalated cells express H+-ATPase in the basolateral plasma membrane and in vesicles throughout the cytoplasm, but band 3 was not detected in the apical membrane. Non-A/non-B cells were characterized by the presence of H+-ATPase in the apical plasma membrane, but the cells did not show band 3 immunoreactivity (Fig. 2).
With immunoperoxidase labeling, band 3 immunoreactivity was seen only in type A cells, where it was located in the basolateral plasma membranes in the CNT, CCD, and OMCD (Fig. 3). The basal staining was located on the numerous small vesicles, some of which were located in the Golgi region (Fig. 3). There was no labeling of mitochondria or of the apical membrane in any cells of the CNT, CCD and OMCD. The luminal plasma membrane showed no evidence of specific immunolabeling, and band 3 immunoreactivity was not observed in type B cells (data not shown). Immunoreactivity for band 3 was greater in the CCD and OMCD cells than in the CCD cells (Fig. 3).
The mammalian collecting duct is capable of both reabsorption and secretion of bicarbonate, depending on the acid-base status of the animal [10]. The mammalian collecting duct is composed of two structurally and functionally distinct cell types-principal cells and intercalated cells [16]. Intercalated cells play a major role in proton and bicarbonate secretion in the collecting duct. These cells constitute between 30% and 40% of the cells in the CNT, CCD, and OMCD [10,16].
A recent study characterized the differentiation of intercalated cells in the rat, mouse, and rabbit kidney [2,14]. Type A intercalated cells secrete protons mediated by a vacuolar type H+-ATPase, which is located in the apical plasma membrane and apical tubulovesicles [15]. They reabsorb HCO3- via a truncated form of the erythrocyte Cl-/HCO3- exchanger, band 3, which is located in the basolateral plasma membrane [2,14]. Type B intercalated cells secrete HCO3- via an apical Cl-/HCO3- exchanger that is functionally distinct from the basolateral Cl-/HCO3- exchanger in type A intercalated cells [3,11]. Type B intercalated cells express H+-ATPase in the basolateral plasma membrane and in vesicles throughout the cytoplasm [15]. Non-A/non-B cells have been described in the CNT and CCD of both the mouse [4,15] and the rat kidney [4,9]. They are characterized by the presence of H+-ATPase in the apical plasma membrane and pendrin in the basolateral plasma membrane [17].
This study was designed to investigate the subcellular distribution of the band 3 protein through the tubular segment including the CNT, CCD and OMCD. This study was the first characterization of band-3-positive intercalated cells in the kidney of the common marmoset monkey, Callithrix jacchus. Type A intercalated cells, identified by the presence of apical H+-ATPase and basolateral band 3 immunostaining, constitute a major proportion of the intercalated cells in the CNT, CCD, and OMCD of the marmoset kidney. In this study, we used specific antibodies directed against H+-ATPase and band 3 protein to characterize the localization of the intercalated cells in the marmoset kidney. The results of the present study demonstrate that band 3 is expressed in the basolateral plasma membranes of all type A intercalated cells in the CNT, CCD, and OMCD of the marmoset kidney. This demonstration of band 3 expression agrees with the observations of Alper et al. [1] and provides further support for the notion that band 3 defines the basolateral plasma membrane of type A intercalated cells [1]. However, electron microscopy confirmed that band 3 immunolabeling of the basolateral plasma membrane was more intense in the CCD and OMCD, whereas immunolabeling of intracellular vesicles was more intense in the CNT. In addition, we found that there was no immunolabeing in the mitochondria of type A interacted cells. From these observations, we suggest that type A interacted cells of the CNT accumulate band 3 protein in small transport vesicles in the inactivated state in the monkey kidney.
Band 3 is the basolateral Cl/HCO3 exchanger of the acid secreting Type A intercalated cell. Distal renal tubular acidosis is characterized by defective acid secretion by the type A interacted cells [1]. This study has demonstrated that band 3 is expressed in the basolateral plasma membranes of type A intercalated cells and has a role in the acid-base balance in the marmoset kidney.
In summary, band 3 in the monkey kidney was present in the basolateral membrane and was present in the intracellular vesicles of type A intercalated cells, and has a role in acid-base balance, especially in the CCD and OMCD.
Figures and Tables
Fig. 1
Light micrographs of 50 µm thick vibratome sections showing immunostaining for band 3 in the cortex (A, C) and outer medulla (B, D) of the marmoset kidney. A: In the cortex of the marmoset kidney, band 3 immunoreactivity was evident in the connecting tubule (CNT) and cortical collecting duct (CCD). B: In the outer medulla of the marmoset monkey kidney, band 3 immunoreactivity was evident in the outer medullary collecting duct (OMCD). C, D: Higher magnification of A and B, respectively. Scale bar = 100 µm.
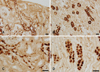
Fig. 2
Differential interference contrast (DIC) microscopy of 1 µm sections of the CNT and CCD (A) and OMCD (B) of the marmoset kidney. Double immunolabeling for H+-ATPase (blue) and band 3 (brown) in 1 µm sections shows that band 3 is expressed in the basolateral plasma membrane of H+-ATPase-positive intercalated cells. Arrowheads, type A intercalated cells with basolateral band 3 and apical H+-ATPase in the CNT, CCD, and OMCD. Arrows, type B intercalated cells with basolateral H+-ATPase in the CNT and CCD. Stars, non-A/non-B cells with apical H+-ATPase in the CNT and CCD. Scale bar = 100 µm.

Fig. 3
Transmission electron micrographs showing band 3 immunoreactivity in the CNT (A), CCD (B), and OMCD (C) of the marmoset kidney. Band 3 immunoreactivity is evident along the basolateral membrane and in vesicles in the basolateral parts of the cytoplasm (A-C). The intensity of the band 3 immunoreactivity of the basolateral plasma membrane in the CCD and OMCD cells is stronger than that in the CNT cells in A. Scale bar = 2.5 µm.

References
1. Alper SL, Darman RB, Chernova MN, Dahl NK. The AE gene family of Cl/HCO3- exchangers. J Nephrol. 2002. 5:S41–S53.
2. Drenckhahn D, Schlüter K, Allen D, Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985. 230:1287–1289.

3. Ercolani L, Brown D, Stuart-Tilley A, Alper SL. Colocalization of GAPDH and band 3 (AE1) proteins in rat erythrocytes and kidney intercalated cell membranes. Am J Physiol. 1992. 262:F892–F896.

4. Kim J, Tisher CC, Linser PJ, Madsen KM. Ultrastructural localization of carbonic anhydrase II in subpopulations of intercalated cells of the rat kidney. J Am Soc Nephrol. 1990. 1:245–256.

5. Kim J, Welch WJ, Cannon JK, Tisher CC, Madsen KM. Immunocytochemical response of type A and type B intercalated cells to increased sodium chloride delivery. Am J Physiol. 1992. 262:F288–F302.

6. Kopito RR. Molecular biology of the anion exchanger gene family. Int Rev Cytol. 1990. 123:177–199.

7. Kudrycki KE, Newman PR, Shull GE. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 Cl-/HCO3- exchanger. J Biol Chem. 1990. 265:462–471.

8. Madsen KM, Brenner BM. Tisher CC, Brenner BM, editors. Structure and function of the renal tubule and the interstitium. Renal Pathology with Clinical and Functional Correlations. 1994. Philadelphia: Lippincott;661–698.
9. Madsen KM, Verlander JW, Kim J, Tisher CC. Morphological adaptation of the collecting duct to acid-base disturbances. Kidney Int Suppl. 1991. 33:S57–S63.
10. Madsen KM, Kim J, Tisher CC. Intracellular band 3 immunostaining in type A intercalated cells of rabbit kidney. Am J Physiol. 1992. 262:F1015–F1022.

11. Muto S, Yasoshima K, Yoshitomi K, Imai M, Asano Y. Electrophysiological identification of α- and β-intercalated cells and their distribution along the rabbit distal nephron segments. J Clin Invest. 1990. 86:1829–1839.

12. Nissant A, Paulais M, Lachheb S, Lourdel S, Teulon J. Similar chloride channels in the connecting tubule and cortical collecting duct of the mouse kidney. Am J Physiol Renal Physiol. 2006. 290:F1421–F1429.

13. Ostedgaard LS, Jennings ML, Karniski LP, Schuster VL. A 45-kDa protein antigenically related to band 3 is selectively expressed in kidney mitochondria. Proc Natl Acad Sci USA. 1991. 88:981–985.

14. Schuster VL, Fejes-Toth C, Naray-Fejes-Toth A, Gluck SL. Colocalization of H+-ATPase and band 3 anion exchanger in rabbit collecting duct intercalated cells. Am J Physiol Renal Physiol. 1991. 260:F506–F517.
15. Tisher CC, Madsen KM. Brenner BM, Rector FC, editors. Anatomy of the kidney. The Kidney. 1991. 4th ed. Saunders: New York;3–75.
16. Verlander JW, Madsen KM, Tisher CC. Axial distribution of band 3-positive intercalated cells in the collecting duct of control and ammonium chloride-loaded rabbits. Kidney Int Suppl. 1996. 57:S137–S147.
17. Verlander JW, Madsen KM, Low PS, Allen DP, Tisher CC. Immunocytochemical localization of band 3 protein in the rat collecting duct. Am J Physiol Renal Physiol. 1988. 255:F115–F125.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download