Abstract
Phosphorylation of caveolin-1 occurs during cell activation by various stimuli. In this study, the involvement of caveolin-1 in an irradiation injured spinal cord was examined by analyzing the phosphorylation of caveolin-1 in the spinal cord of rats after irradiation with a single dose of 15 Gray from a 60Co γ-ray source at 24 h post-irradiation (PI). A Western blot analysis showed that the phosphorylated form of caveolin-1 (p-caveolin-1) was expressed constitutively in the normal spinal cords and was significantly higher in the spinal cord of irradiated rats at 24 h PI. The increased expression of ED1, which is a marker of activated microglia/macrophages, was matched with that of p-caveolin-1. In the irradiated spinal cords, there was a higher level of p-caveolin-1 immunoreactivity in the isolectin B4-positive microglial, ependymal, and vascular endothelial cells, in which p-caveolin-1 was weakly and constitutively expressed in the normal control spinal cords. These results suggest that total body irradiation induces activation of microglial cells in the spinal cord through the phosphorylation of caveolin-1.
Caveolin is a transmembrane adapter molecule that recognizes glycosylphosphatidyl inositol-linked proteins and interacts with downstream cytoplasmic signaling molecules, such as Src-family tyrosine kinases and heterotrimeric G proteins [8,11]. Caveolin is phosphorylated under certain activation conditions, specifically, at Tyr-14, Ser-88, and other residues in v-Src-transformed cells leading to the flattening, aggregation, and fusion of caveolae and caveolae-derived vesicles [8]. Moreover, the phosphorylation of caveolin affects the cell shape, which is an important finding in the activation and migration of inflammatory cells. It was previously reported that the phosphorylation of caveolin-1 occurs with experimental autoimmune encephalomyelitis, particularly in activated microglia or macrophages in the spinal cord [6]. Here, we hypothesize that the phosphorylation of caveolin-1 is an important event for cell activation in central nervous system (CNS) inflammation and possibly irradiation.
Radiation is widely used to treat common central nervous system cancers, particularly glioblastoma multiforme, with or without chemotherapy [1,3] as well as for supportive treatment of a spinal cord injury in animal models [15]. Even though radiation is used for cancer treatment, various side effects can occur, such as glial cell activation, disruption of the blood brain barrier, and white matter necrosis in the CNS [1,3,7,14]. Previous studies suggest that irradiation activates the microglia and eventually induces astrogliosis. Furthermore, a variety of cell activation events, including the increased level of p38 phosphorylation, NF-κB activation, and inflammatory mediators, occur in microglia during irradiation [5]. Little is however known about the cellular responses to irradiation, in particular, the changes to lipid raft proteins such as caveolin-1. The aim of this study is to localize the expression, and examine the changes in the phosphorylation of caveolin-1 (p-caveolin-1) in the spinal cord of rats after irradiation.
Sprague-Dawley rats were purchased from Daehan Biolink (Korea) and bred in our animal facility. Five- to six-week-old male rats weighing 121.1 ± 19.2 g were used for the experiments. The study was carried out in accordance with the internationally accepted principles for laboratory animal use and care as per NIH guidelines (USA).
The rats were divided into two equal groups and anesthetized with chloral hydrate (375 mg/kg body weight, peritoneal injection). The rats from one group were subjected to whole-body irradiation with 15 Gray in a single fraction (n = 7). Irradiation was carried out using a 60Co γ-ray source (10,000 Ci; Co-60 Irradiation Facility, Applied Radiological Science Research Institute, Cheju National University, Korea). The rats from the other group were not irradiated and used as controls (n = 7).
The rats were sacrificed at 24 h post-irradiation (PI) under ether anesthesia. The spinal cords were removed immediately after death and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at pH 7.4 to allow for paraffin embedding, or were quick-frozen and stored for later use in immunoblotting.
Rabbit polyclonal anti-p-caveolin-1 (Tyr 14; Santa Cruz, USA), rabbit polyclonal anti-fibronectin (Santa Cruz, USA), and mouse monoclonal anti-beta-actin (Sigma, USA) antibodies were used in this study. The astrocytes and macrophages were identified by applying the mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibody (Sigma, USA) and mouse monoclonal anti-rat macrophage (ED 1; Serotec, UK), respectively. ED1 was also used to estimate the level of microglia and macrophage activation in a Western blot analysis since ED1 recognizes the rat macrophage lysosomal membrane antigen [2]. Biotinylated isolectin B4 (IB4) derived from Griffonia simplicifolia (Sigma, USA) was used to detect the vascular endothelial cells and activated microglia [6].
A Western blot analysis was performed as previously described [6]. Briefly, the tissue was homogenized in a lysis buffer containing protease and a phosphatase inhibitor. The proteins were resolved by SDS-PAGE (12% acrylamide) and transferred to a nitrocellulose membrane (Schleicher & Schuell, USA). The blots were blocked with 5% skim milk in TTBS (TBS with 0.1% Tween 20) for 1 h, washed, and then incubated with primary antibodies overnight. The blots were washed three times with TTBS and incubated for 1 h with HRP-conjugated anti-rabbit or mouse IgG antibodies (Vector, USA). The immunoreactive bands were developed using a chemiluminescent substrate (WEST-one Kit; iNtRON Biotech, Korea).
To assess the immunohistochemistry, paraffin-embedded spinal cord sections (5 µm) were deparaffinized, treated with a citrate buffer (0.01 M, pH 6.0) in a microwave for 10 min, and then treated with 0.3% hydrogen peroxide in methyl alcohol for 20 min to block the endogenous peroxidase activity. After three washes with PBS, the sections were incubated with 10% normal goat serum and then with polyclonal anti-p-caveolin-1 for 1 h at room temperature (RT). The immunoreactivity was visualized using an avidin-biotin peroxidase complex (Vector Elite; Vector, USA) and the peroxidase reaction was developed using a diaminobenzidine substrate kit (Vector, USA).
The cell phenotype of p-caveolin-1 expression was examined by applying double immunofluorescence using anti-GFAP. The paraffin sections were reacted sequentially with primary rabbit anti-p-caveolin-1 followed by fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG (1 : 50 dilution; Sigma, USA). The sections were then incubated with anti-GFAP followed by tetramethyl rhodamine isothicyanate (TRITC)-labeled goat anti-mouse IgG (1 : 50 dilution; Sigma, USA). To observe the co-localization of p-caveolin-1 and IB4 in the spinal cords, the sections were reacted with biotinylated IB4 (Sigma, USA), followed by TRITC-labeled streptavidin (Zymed, USA). Next, the sections were then reacted with the rabbit anti-p-caveolin-1, followed by a reaction with FITC-labeled goat anti-rabbit IgG (Sigma, USA). For the reduction of lipofuscin autofluorescence, the sections were washed in PBS (three times for 1 h) at RT, dipped briefly in distilled H2O, treated with 10 mM CuSO4 in an ammonium acetate buffer (50 mM CH3COONH4, pH 5.0) for 20 min, dipped again briefly in distilled H2O, and then returned to PBS. Following this, the double immunofluorescence-stained specimens were examined by laser confocal microscopy (FV500; Olympus, Japan).
A Western blot analysis showed that the level of p-caveolin-1 expression was significantly higher in the spinal cord at 24 h PI (0.267 ± 0.036; n = 7 rats; p < 0.05 vs. normal controls) than in the normal controls (density value, 0.066 ± 0.015 OD/mm2; n = 7 rats) (Fig. 1A).
A semiquantitative analysis of the macrophage marker using ED1 was performed to show the activation of microglial cells. The immunoblot of the rat macrophage lysosomal membrane antigen detected by ED1 showed significantly higher levels in the spinal cord at 24 h PI (0.012 ± 0.001; n = 7 rats; p < 0.05 vs. normal controls) than in the normal controls (0.004 ± 0.001 OD/mm2; n = 7 rats) (Fig. 1B).
A Western blot analysis was used to measure the level of fibronectin in order to determine if there was any leakage of blood fibronectin in the spinal cords with irradiation. The level of fibronectin immunoreactivity was significantly higher in the irradiated spinal cords at day 1 PI (0.927 ± 0.068; n = 7 rats; p < 0.05 vs. normal controls) than in the normal control spinal cords (0.436 ± 0.027 OD/mm2; n = 7 rats) (Fig. 1C).
Weak p-caveolin-1 immunoreactivity was constitutively detected in some vascular endothelial, glial, and ependymal cells in the spinal cords of the normal rats (Figs. 2A & B). In the irradiated spinal cords, p-caveolin-1 was found to be intensely immunostained in the ramified glial cells as well as in some vascular endothelial and ependymal cells (Figs. 2C & D).
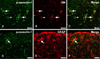
A double-labeling experiment was performed in the spinal cords to determine the cell phenotype of p-caveolin-1 expression. The immunoreactivity of p-caveolin-1 in the parenchyma was co-localized in some IB4-positive microglia and vascular endothelial cells (Figs. 3A-C) but was scarce in the astrocytes (Figs. 3D-F). This suggests that most p-caveolin-1 positive cells are ramified microglial cells.
This is the first study demonstrating an increase in the level of caveolin-1 phosphorylation in irradiated rats located primarily in the microglial and vascular endothelial cells of the spinal cord. The findings suggest that the increased phosphorylation of caveolin-1 indicated that it plays an important role in the cellular changes of CNS tissue after irradiation. Although the biological relevance of caveolin-1 phosphorylation in microglial and vascular endothelial cells remains to be determined, there is a general consensus that it may stimulate a downstream element of p38 mitogen-activated protein kinase and c-Src in NIH3T3 cells [13].
Previous studies have identified the events leading to microglial activation, including increased p38 phosphorylation, NF-κB activation, and release of inflammatory mediators [5]. In addition, a previous study reported that the phosphorylation of caveolin-1 leads to the up-regulation of CD86 in monocytes through NF-κB activation [10]. It is a logical explanation to state that the increased phosphorylation of p38 and NF-κB activation in the microglia are associated with the phosphorylation of caveolin-1 in the spinal cord of irradiated rats, leading to the activation of microglia.
Furthermore, gamma irradiation induces inflammation in the CNS, which is characterized by the activation of microglia, and this cell is in turn affected by pro-inflammatory cytokines [7]. Microglia respond to gamma irradiation through the significant up-regulation of IL-1β and TNFα mRNA synthesis at 4 h and 24 h in vitro [7]. IL-1β induces the phosphorylation of caveolin-1 in HIT-T15 cells [12]. Therefore, it is highly probable that IL-1β induces the phosphorylation of caveolin-1 in the microglia, leading to the inflammatory events after irradiation.
Fibronectin is a component of the extracellular matrix that may leak through the blood-brain barrier and as a result play an important role in activating the cell. Friedrich et al. [4] reported that the level of fibronectin increased after irradiation. Moreover, Milner and Campbell [9] showed an association between fibronectin and the activation of microglial cells. Therefore, we postulate that the increased leakage of fibronectin in the spinal cord after irradiation plays some role in the activation of microglial cells.
Overall, this study demonstrates that the irradiation induces the activation of microglial cells, possibly through the phosphorylation of caveolin-1. The significance of increased phosphorylation in the microglia of CNS tissue after irradiation remains to be determined and should be addressed in future research efforts.
Figures and Tables
Fig. 1
Western blot analysis for p-caveolin-1 (A), ED1 (B), fibronectin (C) in the spinal cords of the normal control rats (Normal controls) and irradiated rats at day 1 post-irradiation (Irradiation). The photographs represent the Western blot for p-caveolin-1 (A), ED1 (B), fibronectin (C) and beta-actin. The p-caveolin-1, ED1, and fibronectin immunoreactivities were detected at low levels in the spinal cords of the normal controls (n = 7) and were found to be significantly greater at day 1 post-irradiation (n = 7; p < 0.05). The arrowheads indicate the positions of p-caveolin-1 (approximately 22 kDa), ED1 (110 kDa), fibronectin (220 kDa) and beta-actin (45 kDa).
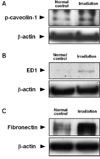
Fig. 2
Immunohistochemical staining of p-caveolin-1 in the spinal cord of the normal control (A, B) and irradiated rats at 24 h post-irradiation (C, D). p-caveolin-1 was weakly detected in some glial (A, arrow), vascular endothelial (A, arrowhead), and ependymal cells (B, arrow) in the normal control spinal cords. Conversely, intense p-caveolin-1 immunoreactivity was detected in the ramified glial (C, arrows), vascular endothelial (C, arrowheads), and ependymal cells (D, arrow) from the irradiated spinal cords which were counterstained with hematoxylin. Scale bars = 30 µm.
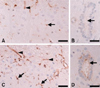
Fig. 3
Identification of p-caveolin-1-positive cells in the spinal cord of the irradiated rats at 24 h post-irradiation. The immunoreactivity of p-caveolin-1 (A, green) was co-localized in some isolectin B4-positive microglia (arrows) and some vascular endothelial cells (arrowhead) in the parenchyma (B, red) (C, merge). Some p-caveolin-1-positive cells (D, green, arrow) tested positive for scarce amounts of glial fibrillary acidic protein GFAP (E, red, arrow) in the white matter (F, merge, arrow). Scale bars = 20 µm.
Acknowledgments
This work was supported by a Program of the Basic Atomic Energy Research Institute (BAERI), which is a part of the Nuclear R&D Programs funded by the Ministry of Science & Technology (MOST) of Korea.
References
1. Burger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer. 1985. 56:1106–1111.

2. Damoiseaux JG, Döpp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994. 83:140–147.
3. Fisher BJ, Scott C, Macdonald DR, Coughlin C, Curran WJ. Phase I study of topotecan plus cranial radiation for glioblastoma multiforme: results of Radiation Therapy Oncology Group Trial 9507. J Clin Oncol. 2001. 19:1111–1117.

4. Friedrich RE, Bartel-Friedrich S, Holzhausen HJ, Lautenschläger C. The effect of external fractionated irradiation on the distribution pattern of extracellular matrix proteins in submandibular salivary glands of the rat. J Craniomaxillofac Surg. 2002. 30:246–254.

5. Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, Ji KA, Joe EH, Cho KH, Han IO. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 2006. 21:457–467.

6. Kim H, Ahn M, Lee J, Moon C, Matsumoto Y, Koh CS, Shin T. Increased phosphorylation of caveolin-1 in the spinal cord of Lewis rats with experimental autoimmune encephalomyelitis. Neurosci Lett. 2006. 402:76–80.

7. Kyrkanides S, Olschowka JA, Williams JP, Hansen JT, O'Banion MK. TNF alpha and IL-1beta mediate intercellular adhesion molecule-1 induction via microglia-astrocyte interaction in CNS radiation injury. J Neuroimmunol. 1999. 95:95–106.

8. Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996. 271:3863–3868.
9. Milner R, Campbell IL. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003. 170:3850–3858.

10. Ohnuma K, Yamochi T, Uchiyama M, Nishibashi K, Yoshikawa N, Shimizu N, Iwata S, Tanaka H, Dang NH, Morimoto C. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc Natl Acad Sci USA. 2004. 101:14186–14191.

11. Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. J Biol Chem. 1998. 273:5419–5422.

12. Veluthakal R, Chvyrkova I, Tannous M, McDonald P, Amin R, Hadden T, Thurmond DC, Quon MJ, Kowluru A. Essential role for membrane lipid rafts in interleukin-1beta-induced nitric oxide release from insulin-secreting cells: potential regulation by caveolin-1+. Diabetes. 2005. 54:2576–2585.

13. Volonté D, Galbiati F, Pestell RG, Lisanti MP. Cellular stress induces the tyrosine phosphorylation of caveolin-1 (Tyr(14)) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J Biol Chem. 2001. 276:8094–8103.

14. Wong CS, Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv. 2004. 4:273–284.

15. Zhang SX, Geddes JW, Owens JL, Holmberg EG. X-irradiation reduces lesion scarring at the contusion site of adult rat spinal cord. Histol Histopathol. 2005. 20:519–530.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download