Abstract
This study examined the suitability of a nuclear imaging technique using 99mTc-tetrofosmin as an agent to assess the heart functions of healthy micropigs. The mean age of the pigs was 360 days (male), and the mean body weight was 35.3 kg ranging from 34.5-36 kg. There were no significant perfusion defects in any of the reconstructed images. Gated single-photon emission computed tomography imaging can be used to calculate the ventricular volume and ejection fraction (EF). In this case, an EF of 79% was calculated from the ventricular volume of the end-systolic image (10 ml) subtracted from that of the end-diastolic volume (49 ml). A perfusion defect (particularly the apex, lateral wall) is unlikely because of the presence of a preserved wall motion in a segment with a defect. It is concluded that quantitative cardiac scintigraphy, using 99mTc-tetrofosmin is an adequate technique for estimating the heart functions of healthy micropigs.
Transplantation is the preferred treatment for chronic failure of the heart, kidneys, lungs, and liver. However, transplantation has a fairly limited impact on medical practice due to the lack of organs. It has been estimated that <10% of the organs needed each year in the United States become available, and that the percentage is continuing to decline [3]. Hence, there is increasing interest in the possibility of using animals as a source of organs and tissues for xenotransplantation in place of humans. The use of animals as a source of organs also might allow the transplant procedure to be planned, providing obvious medical and surgical benefit. The use of animals also might allow the transplant to become a means for expressing extrinsic genes, i.e., as a vehicle for gene transfer.
The species that might best serve as a source of organs or tissues for xenotransplantation is an important issue. The most suitable source of organs and its tissues might intuitively be species such as baboons or other higher primates genetically similar to humans. Indeed, some of the earliest trials in allotransplantation involved the use of organs from higher primates [13,14]. It is possible given modern approaches to immunosuppression and the availability of antimicrobial therapy that organs from nonhuman primates might be made to survive and function for a significant period in human subjects. Most researchers are looking to the use of non-primates such as pigs as source of xenograft tissues and organs for clinical purposes. The reason for this interest is that these animals are available in sufficient numbers and can meet the potential needs of all recipients: 1) pigs can be obtained in sizes suitable for mature adults; 2) pigs present a much lower risk introducing lethal viral infections into the recipient than nonhuman primates; 3) pigs can be genetically engineered. There is only limited information on how well a porcine heart might address this question.
It is clinically impractical to measure the myocardial viability in most animals used for heart transplantation, in which a frequent assessment of the heart functions is essential for both diagnosis and prognosis. An assessment of the myocardial viability in pigs with coronary artery disease and left ventricle dysfunction is of major importance for the prognosis. Pigs with this condition are at high risk of cardiac death and usually have significant impairment in their exercise capacity and daily activity. It is well known that a left ventricle dysfunction is not necessarily an irreversible process. A dysfunctional but viable myocardium has the potential to recover after the restoration of myocardial blood flow by either coronary arterial bypass grafting or percutaneous transluminal coronary angioplasty. Therefore, the aim of this study was to determine the ejection fraction (EF) of healthy micropigs using nuclear imaging with 99mTc- tetrofosmin.
Mixed-breed, conditioned micropigs were purchased from PWG Genetics (Korea). Prior to purchase, physical examinations were performed and their results were considered to be normal. The pigs were housed indoors individually in cages, fed dry pig food, and provided water ad libitum. The age of the experimental animals was 360 days, respectively. Blood pressure was measured five times for each individual animal with Cardell BP monitor (Sharn Veterinary, USA) in the premedicated condition with atropine (0.04 mg/kg IM), xylazine (2.2mg/kg IM), and a zolazepam/tiletamine cocktail (4.4 mg/kg IM) prior to echocardiographic examination (Table 1).
The micropigs were fasted overnight, premedicated with atropine (0.04 mg/kg IM), xylazine (2.2 mg/kg IM), and anesthetized with a zolazepam/tiletamine cocktail (4.4 mg/kg IM). 99mTc-tetrofosmin 111 MBq was injected intravenously at rest. Forty minutes after the injection, the planar and single-photon emission computed tomography (SPECT) images were acquired in the supine position (Fig. 1). The acquisition time was 20 seconds with detection every 3°. The remaining SPECT study was acquired with an ECG-gated technique using eight frames for a cardiac cycle. The SPECT data was acquired using a Discovery VH/Millenium VG (GE Medical System, USA) with a high-resolution collimator, setting the energy photo-peak at 140 keV with a 20% symmetric window and a 90° acquisition arc. The Emory Cardiac Tool Box program automatically reoriented the transaxial slices along the vertical long axis, the horizontal long axis and the short axis [17]. The end-diastolic (ED) and end-systolic (ES) images were selected according to the left ventricular cavity size on the gated SPECT. A polar map was also obtained for all sets. When displayed, the images were normalized to the maximal activity registered during the diastole, systole, or during the overall cardiac cycle. The perfusion defects were assigned to the vascular territories as follows: defects located in the apex, anterior, anterolateral, anteroseptal, septal, and inferoseptal segments were assigned to the left anterior descending artery territory, the inferior segment was assigned to the right coronary artery, and the rest (i.e., lateral and inferolateral segments) were assigned to the left circumflex artery territories. The defect severity was evaluated using a semiquantitative five-point scoring system (0, normal perfusion; 1, mild reduction in counts but not definitely abnormal; 2, moderate reduction in counts and definitely abnormal; 3, severe reduction in counts; 4, absent uptake).
The short-axis (Fig. 2A), vertical long-axis (Fig. 2B) and horizontal long-axis (Fig. 2C) images were displayed. There were no significant perfusion defects in any of the reconstructed images. Gated SPECT imaging allows the ventricular volumes and EF to be calculated (Fig. 3). In this case, an EF of 79% was calculated from the ventricular volume of the ES image (10 ml) on the left subtracted from that of the ED volume (49 ml) on the right. The ES images below show excellent myocardial thickening compared with the ED images shown in Fig. 4A. As shown in Table 2, the mild to moderate defect was observed in Pig 3, but a perfusion defect (especially, apex, lateral wall) is unlikely because of the presence of a preserved wall motion in a segment with a defect [2].
99mTc-tetrofosmin is a lipophilic, cationic diphosphine, and the more recent of the technetium-labeled perfusion tracers for a diagnosis and risk stratification of coronary artery disease (CAD). It enters myocardial cells through passive transport driven by the negative membrane potential of the intact cell. Once within the myocardium it is localized mainly within the cytosol and only a fraction passes into the mitochondria [1,11,18]. The biological half-life for the normal myocardium is approximately 5 h. The blood clearance of tetrofosmin is quite rapid; <5% of the residual activity is present after 10 min. Although the diagnostic sensitivity in mild to moderate CAD appears to be similar, tetrofosmin does not undergo significant redistribution from its initial pattern of uptake. Therefore, imaging can be performed for up to 4 h after the injection. There are only two important differences between sestamibi and tetrofosmin. The first is that the preparation of tetrofosmin does not require a boiling step. Second, perhaps more importantly, the hepatic clearance of tetrofosmin is more rapid [4,10].
Myocardial SPECT (M- SPECT) is a well-established and important technique for the diagnosis and risk stratification of patients with CAD [6-9,12,16]. Millions of studies are performed each year, highlighting the significant clinical need addressed by this methodology. For patients with radionuclide evidence of ischemia, the positive predictive value of such testing is uniformly low, in the range of 4% to 20%. However, the negative predictive value of a normal scan is very high (96% to 100%). The positive predictive value of perfusion imaging can be improved when testing is applied selectively to patients with a higher pretest likelihood of CAD and when the results are integrated into a clinical risk assessment [8]. M-SPECT adds incremental prognostic information when used in patients who have not undergone prior catheterization or revascularization and have not had any previous myocardial infarctions and are at overall low to intermediate risk (1.8% hard event rate, 1.2% per year of follow-up). Furthermore, veterinarians refer patients to catheterization and revascularization according to the extent and severity of their scan results, and hence, to their risk of cardiac events. Therefore, the effect of testing on patient management appears to be both powerful and appropriate [5].
Myocardial perfusion imaging showed a higher sensitivity for detecting disease (92%), as well as a higher specificity for ruling out the presence of disease (80%) [15]. Gated SPECT imaging has significantly improved the practice of nuclear cardiology with respect to assessing the cardiac function and artifact determination. The counts detected during other periods of the cardiac cycle, as determined by ECG timing, will be applied to the corresponding image showing a particular moment in the cardiac cycle. These static images represent the degree of ventricular thickening during a single cardiac cycle. The degree of ventricular thickening and EF can be calculated if these images are run in a continuous loop. The proper identification of artifacts is a major advantage of gated SPECT imaging. DePuey et al. [2] suggested that the presence of a preserved wall motion in a segment with a fixed defect is indicative of an artifact rather than an infarct. They reported that this type of analysis could reduce the false-positive rate significantly. In conclusion, quantitative cardiac scintigraphy using 99mTc-tetrofosmin is a good technique for estimating the heart functions of healthy micropigs.
Figures and Tables
Fig. 1
A micropig positioned ventro-dorsally, and restrained physically and pharmacologically for an assessment of the cardiac function using gated 99mTc-tetrofosmin single photon emission computed tomography.
Fig. 2
Cardiac perfusion images. A-C: These images showed myocardial perfusion (Yellow arrow: anterior wall, Yellow dotted arrow: septal wall, white arrow: inferior wall, white dotted arrow: lateral wall). Cardiac short-axis (A), vertical long-axis (B), horizontal long-axis (C) was represented. The polar map (D) an image showing quantified values of perfusion of each cardiac region as a map.

Fig. 3
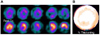
These images represent the cardiac wall motion reconstructed as 3 dimensional image. The cardiac wall motion images showed a visualization of the radioactivity of 99mTc-tetrofosmin in the heart and cardiac perfusion volume. A: end-diastole volume B: end-systole volume.
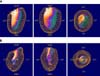
Fig. 4
Images representing the quantitative wall motion and thickening that provides evidence of the ventricular function. A: thickening (above: end-diastolic image, below: end-systolic image), B: thickening map.
Acknowledgments
The authors wish to thank Mr. Jung-Jin Rho for his technical support in acquiring the cardiac scintigraphic images and data analysis. This work was supported by a grant (code # 20070401034006) from BioGreen 21 Program, Rural Development Administration, Republic of Korea. The authors acknowledge a graduate fellowship provided by the Ministry of Education and Human Resources Development through the Brain Korea 21 project, Korea.
References
1. Arbab AS, Koizumi K, Toyama K, Arai T, Araki T. 99mTc-tetrofosmin, 99mTc-MIBI and thallium-201 uptake in rat myocardial cells. J Nucl Med. 1998. 39:266–271.
2. DePuey EG, Rozanski A. Using gated 99mTc-sestamibi SPECT to characterize fixed myocardial defects as infarct or artifact. J Nucl Med. 1995. 36:952–955.
3. Evans RW, Orians CE, Ascher NL. The potential supply of organ donors. An assessment of the efficacy of organ procurement efforts in the united states. JAMA. 1992. 267:239–246.

4. Flamen P, Bossuyt A, Franken PR. 99mTc-tetrofosmin in dipyridamole-stress myocardial SPECT imaging: intraindividual comparison with 99mTc-sestamibi. J Nucl Med. 1995. 36:2009–2015.
5. Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996. 93:905–914.

6. Hachamovitch R, Berman DS, Kiat H, Cohen I, Friedman JD, Shaw LJ. Value of stress myocardial perfusion single photon emission computed tomography in patients with normal resting electrocardiograms: an evaluation of incremental prognostic value and cost-effectiveness. Circulation. 2002. 105:823–829.

7. Hachamovitch R, Hayes S, Friedman JD, Cohen I, Shaw LJ, Germano G, Berman DS. Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: what is the warranty period of a normal scan? J Am Coll Cardiol. 2003. 41:1329–1340.

8. Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, O'Gara PT, Carabello BA, Russell RO, Cerqueira MD, St John Sutton MG, DeMaria AN, Udelson JE, Kennedy JW, Verani MS, Williams KA, Antman EM, Smith SC, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American Society for Nuclear Cardiology. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation. 2003. 108:1404–1418.

9. Mark DB, Shaw LJ, Lauer MS, O'Malley PG, Heidenreich P. 34th Bethesda Conference: Task force #5--Is atherosclerosis imaging cost effective? J Am Coll Cardiol. 2003. 41:1906–1917.
10. Münch G, Neverve J, Matsunari I, Schröter G, Schwaiger M. Myocardial 99mTc-tetrofosmin and 99mTc-sestamibi kinetics in normal subjects and patients with coronary artery disease. J Nucl Med. 1997. 38:428–432.
11. Platts EA, North TL, Pickett RD, Kelly JD. Mechanism of uptake of technetium-tetrofosmin. I: Uptake into isolated adult rat ventricular myocytes and subcellular localization. J Nucl Cardiol. 1995. 2:317–326.

12. Pollock SG, Abbott RD, Boucher CA, Beller GA, Kaul S. Independent and incremental prognostic value of tests performed in hierarchical order to evaluate patients with suspected coronary artery disease. Validation of models based on these tests. Circulation. 1992. 85:237–248.

13. Reemtsma K, Mccracken BH, Schlegel JU, Pearl M. Heterotransplantation of the kidney: two clinical experiences. Science. 1964. 143:700–702.

14. Reemtsma K, Mccracken BH, Schlegel JU, Pearl MA, Pearce CW, Dewitt CW, Smith PE, Hewitt RL, Flinner RL, Creech O. Renal heterotransplantation in man. Ann Surg. 1964. 160:384–410.

15. Rozanski A, Berman DS. The efficacy of cardiovascular nuclear medicine exercise studies. Semin Nucl Med. 1987. 17:104–120.

16. Underwood SR, Godman B, Salyani S, Ogle JR, Ell PJ. Economics of myocardial perfusion imaging in Europe-the EMPIRE Study. Eur Heart J. 1999. 20:157–166.

17. Schaefer WM, Lipke CS, Standke D, Kühl HP, Nowak B, Kaiser HJ, Koch KC, Buell U. Quantification of left ventricular volumes and ejection fraction from gated 99mTc-MIBI SPECT: MRI validation and comparison of the Emory Cardiac Tool Box with QGS and 4D-MSPECT. J Nucl Med. 2005. 46:1256–1263.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download