Abstract
Phospholipid hydroperoxide glutathione peroxidase (PHGPx), an antioxidative selenoprotein, is modulated by estrogen in the testis and oviduct. To examine whether potential endocrine disrupting chemicals (EDCs) affect the microenvironment of the testes, the expression patterns of PHGPx mRNA and histological changes were analyzed in 5-week-old Sprague-Dawley male rats exposed to several EDCs such as an androgenic compound [testosterone (50, 200, and 1,000 µg/kg)], anti-androgenic compounds [flutamide (1, 5, and 25 mg/kg), ketoconazole (0.2 and 1 mg/kg), and diethylhexyl phthalate (10, 50, and 250 mg/kg)], and estrogenic compounds [nonylphenol (10, 50, 100, and 250 mg/kg), octylphenol (10, 50, and 250 mg/kg), and diethylstilbestrol (10, 20, and 40 µg/kg)] daily for 3 weeks via oral administration. Mild proliferation of germ cells and hyperplasia of interstitial cells were observed in the testes of the flutamide-treated group and deletion of the germinal epithelium and sloughing of germ cells were observed in testes of the diethylstilbestrol-treated group. Treatment with testosterone was shown to slightly decrease PHGPx mRNA levels in testes by the reverse transcriptionpolymerase chain reaction. However, anti-androgenic compounds (flutamide, ketoconazole, and diethylhexyl phthalate) and estrogenic compounds (nonylphenol, octylphenol, and diethylstilbestrol) significantly upregulated PHGPx mRNA in the testes (p < 0.05). These findings indicate that the EDCs might have a detrimental effect on spermatogenesis via abnormal enhancement of PHGPx expression in testes and that PHGPx is useful as a biomarker for toxicity screening of estrogenic or antiandrogenic EDCs in testes.
Phospholipid hydroperoxide glutathione peroxidase (PHGPx) is an intracellular antioxidant that belongs to the superfamily of selenium-dependent peroxidases. It interacts directly with peroxidized phospholipid and cholesterol and cholesteryl ester, even when they are incorporated into biomembranes and lipoproteins [13,31,35]. There are three different isoforms of PHGPx (cytosolic, mitochondrial, and nuclear), all derived from a single gene [15]. PHGPx is widely expressed and its enzymatic activity has been detected in a variety of tissues, particularly in the endocrine organs including the testis [5]. PHGPx gene expression and enzymatic activity are hormone-dependent. PHGPx activity is influenced by testosterone levels during spermatogenesis [19]. In rat testes, PHGPx is preferentially expressed after puberty. Although PHGPx is essentially absent following hypophysectomy, it can be partially restored by treatment with human chorionic gonadotropin or testosterone [29].
A variety of structurally diverse natural and synthetic chemicals, called endocrine disrupting chemicals (EDCs), have been reported to interfere with the endocrine system. At the cellular level, EDCs can induce 'endocrine disruption' via a number of routes that involve steroid-receptor binding (agonists), blocking of steroid-receptor binding (antagonists), or disruption of the biosynthesis and metabolism of steroids [30]. Estrogenic and androgenic chemicals with similar structures to estrogen and androgen bind to estrogen receptors (ERs) or the androgen receptor (AR) and ultimately alter the normal function of tissues and organs [16]. ERs and AR are expressed in a cell-specific manner in male and female reproductive organs [27]. Many studies have reported the detrimental effects of EDCs on the development of reproductive organs. Recently, we have demonstrated that the expression of 3β-hydroxysteroid dehydrogenase, a histochemical marker for Leydig cells in the testes, can be affected by treatment with various EDCs [14]. The administration of vinclozolin, an AR antagonist, during sexual differentiation demasculinizes and feminizes male rat offspring, such that the treated males display a female-like anogenital distance at birth, retained nipples, hypospadias, suprainguinal ectopic testes, a blind vaginal pouch, and small-to-absent sex-accessory glands [11]. Neonatal injection of vinclozolin at 200 mg/kg/day demasculinized aggressive play behavior in male rats at 35 days-of-age, indicating that sexual differentiation was altered in an anti-androgenic manner [12]. These findings suggest that reproductive organs are highly susceptible to EDC exposure during organ development and sexual differentiation and that EDCs have the potential to perturb steroidogenesis in testes.
Brigelius-Flohe et al. [6] reported that the porcine PHGPx gene contains a variety of putative regulatory elements, including estrogen-, progesterone-, and glucorticoid-responsive sequences [6]. Recently, it was reported that PHGPx expression and enzymatic activity are up-regulated by estradiol in the female reproductive tract [17]. We have previously demonstrated that estradiol can increase PHGPx mRNA expression in male reproductive organs [23]. In this study, we examine whether potential anti-androgenic or estrogenic EDCs affect spermatogenesis, by analyzing PHGPx mRNA expression and histopathological changes in testes of rats exposed to various EDCs.
Four-week-old male Sprague-Dawley rats were purchased from Samtaco (Korea) and acclimated in polycarbonate cages for 1 week. The animals (n = 10/group) were housed in an environmentally controlled room with a 12-h light/dark cycle, temperature of 21 ± 2℃, and frequent ventilation at 10 times/h. The animals were fed standard rat chow (Samyang, Korea) and tap water ad libitum throughout the experimental period. Testosterone propionate (50, 200, and 1,000 µg/kg), flutamide (FM; 1, 5, and 25mg/kg), diethylhexyl phthalate (DEHP; 10, 50, and 250 mg/kg), ketoconazole (KC; 0.2 and 1 mg/kg), octylphenol (OP; 10, 50, and 250 mg/kg), nonylphenol (NP; 10, 50, 100, and 250 mg/kg), or diethylstilbestrol (DES; 10, 20, and 40 µg/kg) were orally administered to the rats daily for 3 weeks. All chemicals were obtained from Sigma (USA). Control animals received corn oil (the vehicle) for the same period. All animal experiments were conducted in compliance with 'Guide for Care and Use of Animals' (Chungbuk National University Animal Care Committee, according to NIH #86-23).
The rats were euthanized at 8 weeks-of-age under pentobarbital anesthesia and their testes were rapidly removed. Testes were fixed in Bouin's fixative, dehydrated with increasing concentrations of ethyl alcohol, cleared in xylene, infiltrated with paraffin and paraplast with an automatic tissue processor (Shandon, USA), and embedded in paraffin wax with an embedding machine (Leica, Germany). The tissue blocks were cut into 5-µm thicknesses with a rotary microtome (Leica, Germany), stained with hematoxylin and eosin, and observed under a light microscope (Leica, Germany).
Total RNA was extracted from testes using a TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions. The RNA pellet obtained in the final step was dissolved in 50 µl of sterile diethylpyrocarbonate (DEPC)-treated water, and its concentration was determined using a UV spectrophotometer at 260 nm. RNA was kept in DEPC-treated water at -70℃ until use. Total RNA (5 µg) was reverse transcribed using pd(N)6 primers and first-strand cDNA synthesis reagents (Amersham Biosciences, UK). The following primer sets were used to amplify PHGPx (accession number: NM_008162, S1-As2 fragment; 461 bp) and beta-actin (accession number: NM_007393, S1-As2 fragment; 376 bp) as an internal control:
PHGPx forward: 5'-ATGCACGAATTCTCAGCCAAG-3'
PHGPx reverse: 5'-GGCAGGTCCTTCTCTAT-3'
Beta-actin forward: 5'-CGTGACATCAAAGAGAAGCTGTGC-3'
Beta-actin reverse: 5'-GCTCAGGAGGAGCAATGATCTTGAT-3'.
The PCR products were separated on a 2%-agarose gel in Tris-borate-EDTA buffer. Results were analyzed with an AlphaEase V5.5 analyzer system (Alpha Innotech, USA).
The seminiferous tubules in the testosterone-, OP-, NP-, DEHP-, and KC-treated groups showed a normal morphology as well as the control group (Fig. 1A, B & D). However in the testes from the FM-treated group, mild proliferation of germ cells and hyperplasia of interstitial cells were observed (Fig. 1C). In addition, deletion of germinal epithelium and sloughing of germ cells were observed in the group treated with 40 µg/kg/day DES (Fig. 1E & F).
The detrimental effects of EDCs on rat testes were studied using PHGPx mRNA expression as a biomarker. Treatment with EDCs other than testosterone resulted in a general increase in PHGPx expression. The testosterone treatment slightly decreased the PHGPx mRNA levels, but the levels were not significantly different from the control (Fig. 2). The expression of PHGPx mRNA in testes was increased in a dose-dependent manner by FM treatment and was significantly increased to 194% of the control in rats treated with 25 mg/kg/day FM (p < 0.05; Fig. 3). In the DEHPtreated groups, PHGPx mRNA was increased to 113-137% of the control level (Fig. 4). In the KC-treated groups, PHGPx mRNA was significantly increased to 147-150% of the control (p < 0.05; Fig. 5). OP caused the up-regulation of PHGPx mRNA by more than 37% of the control level (Fig. 6). NP stimulated PHGPx mRNA expression in the testes at all doses. In particular, the level was significantly increased to 158% of the control level in the groups treated with 50 and 250 mg/kg/day NP (p < 0.05; Fig. 7). The testicular level of PHGPx mRNA in the rats exposed to various concentrations (10, 20, and 40 µg/kg/day) of DES was significantly higher (154-248%) than that of the control group (p < 0.05; Fig. 8).
Although several putative estrogen-responsive elements have been located within the 5'-untranslated region and the first intron of porcine PHGPx [6], the cellular mechanisms that mediate the action of estrogen on PHGPx regulation require further inquiry. We previously reported that PHGPx mRNA expression in male reproductive organs of rats is greatly influenced by treatment with estradiol or tamoxifen [23]. PHGPx expression in oviducts is also up-regulated by estradiol [17]. Based on this previous evidence, we investigated the expression patterns of PHGPx mRNA and morphological changes in rat testes after treatment with various EDCs, including testosterone, anti-androgenic compounds (FM, KC, and DEHP), and estrogenic compounds (NP, OP, and DES). Our results indicate that estrogenic or anti-androgenic EDCs might have a detrimental effect on spermatogenesis via the abnormal enhancement of PHGPx expression in testes.
In adult males, fertility and sexual functions are androgendependent. The role of androgens such as testosterone and their action mechanism in reproduction are well-established. In testes, the AR is localized in nuclei of cells such as Sertoli cells, Leydig cells, and peritubular myoid cells [27]. In mouse testes, PHGPx mRNA is first expressed at 3 weeks-of-age, greatly increases at 8 weeks, and persists at a high level until 80 weeks. According to in situ analysis, PHGPx mRNA is expressed stage-specifically in spermatogenic cells and Leydig cells [24]. In the present study, treatment with testosterone (50, 200 and 1,000 µg/kg/day) slightly decreased PHGPx mRNA expression in testes. Anti-androgens have the potential to perturb male reproductive development and function in humans and experimental animals and they can act via disturbance of the pituitarygonadal axis [3,11,18,22]. FM blocks the negative feedback of testosterone in the hypothalamus and pituitary and induces over-expression of steroidogenic enzymes in the testis by increasing testosterone production [33]. Ohsako et al. [25] reported that serum and testicular testosterone levels were significantly elevated by the administration of FM [25]. In this study, the expression level of PHGPx mRNA was increased by FM treatment (1-25 mg/kg/day) in a dose-dependent manner. Moreover, mild proliferation of germ cells and hyperplasia of Leydig cells were induced by treatment with FM. These results suggest that PHGPx might be intimately involved in the proliferation of spermatogenic cells through the synthesis of androgen in Leydig cells.
In our study, treatment with DEHP (10-250 mg/kg/day) slightly increased PHGPx mRNA expression in testes. Phthalates, including DEHP, are widely used as a plasticizer in the production of plastics. In mammals, DEHP treatment produces developmental and/or reproductive toxicity with a period of susceptibility extending from the fetal to pubertal stages-of-life and induces reproductive-tract malformations in androgen-dependent tissues in male rat offspring [26]. Unlike FM, DEHP does not interact with the AR. Rather, the effects observed in rodents have been associated with a reduction in testosterone synthesis by the fetal testis [9,22,26]. Phthalate treatment interferes with the transcription of several key genes involved in both cholesterol transport and the biosynthesis of testosterone [2,32]. In contrast, KC, a broad-spectrum imidazole antimycotic agent, interferes with the cytochrome P-450 enzyme system and can cause inhibition of microsomal steroidogenesis in Leydig cells [1,21]. In this study, PHGPx mRNA expression was significantly increased (p < 0.05) to 147-150% of the control level in the KC-treated group. These findings indicate that the anti-androgens DEHP and KC induce dysfunction of Leydig cells and stimulation of PHGPx transcription in testes.
There are two types of ERs, ERα and ERβ, which differ in the C-terminal ligand-binding domain and the N-terminal transactivation domain. In testes, ERα is localized in nuclei of Leydig cells, spermatocytes, and round spermatids. ERβ is detected in spermatogenic cells of various stages and in Sertoli cells, suggesting that estrogens directly affect germ cells during testicular development and spermatogenesis [27]. Alkylphenol ethoxylates are widely used as surfactants throughout the world. Their metabolites (NP, OP) are ubiquitous in the environment [10]. OP has been shown to decrease the expression of steroidogenesis factor-1 mRNA in the fetal testis [20]. It has also been found to bind to the ER and to be weakly estrogenic in vitro [34]. NP is also a weakly estrogenic compound. Several studies have reported the adverse effects of NP on the development of the male reproductive tract [4]. In this study, treatment with OP and NP stimulated PHGPx mRNA expression in testes at all doses. In particular, the signal was significantly increased by 50 or 250 mg/kg/day NP treatment. These results show that the alkylphenolic compounds stimulate the expression of PHGPx mRNA in a pattern similar to that found in our previous estradiol-treatment study [14]. DES is a nonsteroidal synthetic estrogen that has been used to prevent miscarriage and premature birth. According to in vivo knockout studies, DES acts through an ERα-mediated mechanism in the male and female reproductive tracts [7,8,28]. In this study, PHGPx mRNA in the testis was significantly up-regulated at various concentrations (10, 20, and 40 µg/kg/day) of DES. In addition, deletion of germinal epithelium and sloughing of germ cells in the testes were detected following DES treatment. These results suggest that, like endogenous estrogen, environmental estrogenic compounds might stimulate PHGPx expression in testes via the ER pathway.
In conclusion, we demonstrate that anti-androgenic and estrogenic EDCs enhance expression of PHGPx mRNA in the testes, suggesting that PHGPx is useful as a biomarker to screen for detrimental effects of exogenous EDCs in testes.
Figures and Tables
Fig. 1
Histological findings in the testes of 8-week-old rats treated with the vehicle alone (A), testosterone (1,000 µg/kg) (B), flutamide (25 mg/kg) (C), diethylhexyl phthalate (250 mg/kg) (D), or diethylstilbestrol (40 µg/kg) (E & F), daily for 3 weeks by oral administration. H&E stain, A-E; ×100, F; ×200.
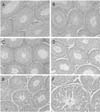
Fig. 2
Analysis of expression levels of PHGPx mRNA in the testes of testosterone-treated rats using reverse transcription-polymerase chain reaction. β-actin: an intrinsic control. The values represent mean ± SD.

Fig. 3
Expression pattern of PHGPx mRNA in the testes of flutamide-treated rats using reverse transcription-polymerase chain reaction. β-actin: an intrinsic control. The values represent mean ± SD. *Significantly different from the control at p < 0.05.

Fig. 4
Investigation of expression levels of PHGPx mRNA in the testes of diethylhexyl phthalate-treated rats using reverse transcription-polymerase chain reaction. β-actin: an intrinsic control. The values represent mean ± SD.

Fig. 5
Expression levels of PHGPx mRNA in the testes of ketoconazole-treated rats. β-actin: an intrinsic control. The values represent mean ± SD. *Significantly different from the control at p < 0.05.

Fig. 6
Analysis of expression levels of PHGPx mRNA in the testes of octylphenol-treated rats using reverse transcription-polymerase chain reaction. β-actin: an intrinsic control. The values represent mean ± SD.

Acknowledgments
This work was supported by a Korea Research Foundation Grant funded by the Korean Government [MOEHRD, Basic Research Promotion Fund (KRF-2005-015-E00218 and KRF-2005-005-J15002)].
References
1. Albertson BD, Frederick KL, Maronian NC, Feuillan P, Schorer S, Dunn JF, Loriaux DL. The effect of ketoconazole on steroidogenesis: I. Leydig cell enzyme activity in vitro. Res Commun Chem Pathol Pharmacol. 1988. 61:17–26.
2. Barlow NJ, Phillips SL, Wallace DG, Sar M, Gaido KW, Foster PM. Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicol Sci. 2003. 73:431–441.

3. Baskin LS, Himes K, Colborn T. Hypospadias and endocrine disruption: is there a connection? Environ Health Perspect. 2001. 109:1175–1183.

4. Boockfor FR, Blake CA. Chronic administration of 4-tert-octylphenol to adult male rats causes shrinkage of the testes and male accessory sex organs, disrupts spermatogenesis, and increases the incidence of sperm deformities. Biol Reprod. 1997. 57:267–277.

5. Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999. 27:951–965.

6. Brigelius-Flohé R, Aumann KD, Blöcker H, Gross G, Kiess M, Klöppel KD, Maiorino M, Roveri A, Schuckelt R, Ursini F, Wingender E, Flohé L. Phospholipid-hydroperoxide glutathione peroxidase. Genomic DNA, cDNA, and deduced amino acid sequence. J Biol Chem. 1994. 269:7342–7348.

7. Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, Korach KS. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol. 2001. 238:224–238.

8. Couse JF, Korach KS. Estrogen receptor-α mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology. 2004. 205:55–63.

9. Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004. 127:305–315.

10. Giger W, Brunner PH, Schaffner C. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science. 1984. 225:623–625.

11. Gray LE, Ostby JS, Kelce WR. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol Appl Pharmacol. 1994. 129:46–52.

12. Hotchkiss AK, Ostby JS, Vandenbergh JG, Gray LE. An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiol Behav. 2003. 79:151–156.

13. Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003. 34:145–169.

14. Kim HH, Kwak DH, Yon JM, Baek IJ, Lee SR, Lee JE, Nahm SS, Jeong JH, Lee BJ, Yun YW, Nam SY. Differential expression of 3 β-hydroxysteroid dehydrogenase mRNA in rat testes exposed to endocrine disruptors. J Reprod Dev. 2007. 53:465–471.

15. Knopp EA, Arndt TL, Eng KL, Caldwell M, LeBoeuf RC, Deeb SS, O'Brien KD. Murine phospholipid hydroperoxide glutathione peroxidase: cDNA sequence, tissue expression, and mapping. Mamm Genome. 1999. 10:601–605.

16. Korach KS, Chae K, Gibson M, Curtis S. Estrogen receptor stereochemistry: ligand binding and hormonal responsiveness. Steroids. 1991. 56:263–270.

17. Lapointe J, Kimmins S, Maclaren LA, Bilodeau JF. Estrogen selectively up-regulates the phospholipid hydroperoxide glutathione peroxidase in the oviducts. Endocrinology. 2005. 146:2583–2592.

18. LeBlanc GA, Bain LJ, Wilson VS. Pesticides: multiple mechanisms of demasculinization. Mol Cell Endocrinol. 1997. 126:1–5.

19. Maiorino M, Wissing JB, Brigelius-Flohé R, Calabrese F, Roveri A, Steinert P, Ursini F, Flohé L. Testosterone mediates expression of the selenoprotein PHGPx by induction of spermatogenesis and not by direct transcriptional gene activation. FASEB J. 1998. 12:1359–1370.

20. Majdic G, Sharpe RM, Saunders PT. Maternal oestrogen/xenoestrogen exposure alters expression of steroidogenic factor-1 (SF-1/Ad4BP) in the fetal rat testis. Mol Cell Endocrinol. 1997. 127:91–98.

21. Miossec P, Archambeaud-Mouveroux F, Teissier MP. Inhibition of steroidogenesis by ketoconazole. Therapeutic uses. Ann Endocrinol (Paris). 1997. 58:494–502.
22. Mylchreest E, Sar M, Cattley RC, Foster PM. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol Appl Pharmacol. 1999. 156:81–95.

23. Nam SY, Baek IJ, Lee BJ, In CH, Jung EY, Yon JM, Ahn B, Kang JK, Yu WJ, Yun YW. Effects of 17β-estradiol and tamoxifen on the selenoprotein phospholipid hydroperoxide glutathione peroxidase (PHGPx) mRNA expression in male reproductive organs of rats. J Reprod Dev. 2003. 49:389–396.

24. Nam SY, Fujisawa M, Kim JS, Kurohmaru M, Hayashi Y. Expression pattern of phospholipid hydroperoxide glutathione peroxidase messenger ribonucleic acid in mouse testis. Biol Reprod. 1998. 58:1272–1276.

25. Ohsako S, Kubota K, Kurosawa S, Takeda K, Qing W, Ishimura R, Tohyama C. Alterations of gene expression in adult male rat testis and pituitary shortly after subacute administration of the antiandrogen flutamide. J Reprod Dev. 2003. 49:275–290.

26. Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000. 58:339–349.

27. Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor α, oestrogen receptor β and androgen receptors in the rat reproductive organs. J Endocrinol. 2000. 165:359–370.

28. Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with αERKO and βERKO mice. Cancer Res. 2001. 61:6089–6097.
29. Roveri A, Casasco A, Maiorino M, Dalan P, Calligaro A, Ursini F. Phospholipid hydroperoxide glutathione peroxidase of rat testis. Gonadotropin dependence and immunocytochemical identification. J Biol Chem. 1992. 267:6142–6146.

30. Sharpe RM, Irvine DS. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? BMJ. 2004. 328:447–451.

31. Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem. 1990. 265:454–461.

32. Thompson CJ, Ross SM, Gaido KW. Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology. 2004. 145:1227–1237.

33. Viguier-Martinez MC, Hochereau-de Reviers MT, Perreau C. Effects of flutamide or of supplementation with testosterone in prepubertal male rats prenatally treated with busulfan. Acta Endocrinol (Copenh). 1985. 109:550–557.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download