Abstract
To examine the involvement of phospholipase D (PLD) isozymes in postnatal testis development, the expression of PLD1 and PLD2 was examined in the mouse testis at postnatal weeks 1, 2, 4, and 8 using Western blot analysis and immunohistochemistry. The expression of both PLD1 and PLD2 increased gradually with development from postnatal week 1 to 8. Immunohistochemically, PLD immunoreactivity was detected in some germ cells in the testis and interstitial Leydig cells at postnatal week 1. PLD was mainly detected in the spermatocytes and residual bodies of spermatids in the testis after 8 weeks after birth. The intense immunostaining of PLD in Leydig cells remained unchanged by postnatal week 8. These findings suggest that PLD isozymes are involved in the spermatogenesis of the mouse testis.
Phospholipase D (PLD), which catalyzes the hydrolysis of phosphatidylcholine to phosphatidic acid (PA) and choline, has been suggested to play an important role in receptormediated signal pathways leading to cell proliferation, differentiation, apoptosis, differentiation, cytoskeletal reorganization, and membrane trafficking and secretory events, possibly including neurotransmitter release [4-6]. PA is generally recognized as the signaling product of PLD and can be further metabolized to diacylglycerol and lysophosphatidic acid by PA phosphohydrolase and phospholipase A2, respectively [2]. Therefore, PLD influences many important intracellular events via the production of these downstream products. Many stimuli regulate PLD activity, including cytokines, growth factors, hormones, neurotransmitters, and other molecules involved in extracellular communication [4-6].
In mammals, two isoforms of PLD, PLD1 and PLD2, have been characterized at the molecular level [8], and many in vitro studies have shown that PLD plays an important role in cell activation, proliferation, and death in various cell culture systems [3,16,18,22]. While information about the cell-specific expression of PLD in tissues [7] may provide clues to the functional relevance of the PLD isozymes, their functional role in cells remains unclear.
PLD has been known to play a role in germ cell development via either cell proliferation or death [5,6]. Recently, we have reported about the expression of PLD in various cell types of mouse testis, including seminiferous germ cells and Leydig cells [10]. Furthermore, PLD is known to play an important role in cell signaling in testis Leydig cells [11,20,21]. Although PLD expression has been examined in the developing retina [13] and hippocampus [17], little is known about the expression and cellular localization of PLD in the testis during postnatal development.
This study examined the expression and cellular localization of PLD in mouse testis at various postnatal time points using Western blot analysis and immunohistochemistry.
Male and female BALB/c mice (25 g, 8-10 weeks old) were obtained from the Choongang Animal Institute (Korea), and bred in our animal facility. Testes (n = 3-5/group) at 1, 2, 4, and 8 weeks after birth were used for Western blot analysis and histology (n = 3-5 samples). All of the experiments were carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (USA).
Testes from mouse were isolated, dissected, and homogenized in lysis buffer for protein analysis. The opposite testis was fixed in 4% paraformaldehyde in phosphate buffer and embedded in paraffin. Paraffin sections were stained with hematoxylin and eosin, and used for immunohistochemistry.
The anti-PLD antibody was generated against the C-terminal 12 amino acid residues 1063-1074 (TKEAIVP MEVWT) of the rat PLD1. The antiserum recognizes both PLD1 and PLD2 both PLD2 and PLD1 because the seven C-terminal amino acids of PLD2 and PLD1 are identical [16]. For affinity purification of the antibodies, the peptide was coupled to Affi-Gel 15 (Bio-Rad, USA) according to the manufacturer's instructions. The anti-PLD antibody used in this study has been shown to label reactive astrocytes and macrophages in rats with EAE [1], ischemic brain injury [14], and clip compression injury [9], as well as ganglion cells and glial cells, including Muller cells, in the developing and adult retina of the rat [12,13].
Tissue samples were homogenized in immunoprecipitation assay buffer [20 mM HEPES (pH 7.2), 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 150 mM NaCl, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 1 mM phenylmethylsulfonylfluoride]. After incubation for 30 min in an ice-bath, the homogenates were centrifuged, and the lysate supernatant was precleared by incubation with preimmune IgG and protein A-Sepharose for 30 min. Precleared cell lysates were incubated with the anti-PLD antibody and 30 µl of the 50% slurry of protein A-Sepharose for 4 h. The immune complex was collected by centrifugation and washed five times with ice-cold buffer that contained 20 mM Tris (pH 7.5), 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, 2 mM Na3VO4, 10% glycerol, and 1% Nonidet P-40. SDS sample buffer was added and the mixture was boiled. The recovered proteins were separated by SDS-polyacrylamide gel electrophoresis. The separated proteins were transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, USA), blocked in 5% nonfat dried milk, and probed with the anti-PLD antibody. Immunoreactive bands were visualized by chemiluminescence using horseradish peroxidase-conjugated secondary antibodies and the ECL reagents (Amersham Biosciences, USA).
Sections of paraffin-embedded tissues (5 µm thick) were deparaffinized and allowed to react with the affinity-purified anti-PLD antibody. Immunoreactivity was visualized with the avidin-biotin peroxidase reaction (Vector Elite; Vector, USA). Peroxidase was developed with diaminobenzidine (Vector, USA). The sections were counterstained with hematoxylin before being mounted. As a control, the primary antibody was omitted for a few test sections, and no specific labelling was found in these sections (Fig. 2C).
To investigate the developmental expression patterns of PLD isozymes in the mouse testis at postnatal ages of 1, 2, 4, and 8 weeks, lysates from mouse testis were immunoprecipitated and analyzed by Western blotting using antibody to PLD (Fig. 1). Both PLD1 and PLD2 were detected in the testis at postnatal week 1, and the expression of two PLD isozymes increased gradually until postnatal week 4 and was enhanced further at postnatal week 8. Considering the initiation of germ cell development around postnatal week 4, PLD expression appears to be closely associated with spermatogenesis.
At 1 (Fig. 2A) and 2 (Fig. 2B) weeks after birth, the seminiferous tubules consist of single or multiple layers of undifferentiated cells. Immunohistochemistry showed weak PLD immunostaining in some undifferentiated cells in the seminiferous tubules. Intense PLD immunostaining was seen in interstitial Leydig cells in the testis of both 1- and 2-week-old mice (Fig. 2A & B).
In the mouse testis at 4 weeks after birth, primary spermatocytes are visualized in the seminiferous tubules. PLD immunoreactivity was visualized in some small primary spermatocytes along the basement membrane (Fig. 2D), where PLD was also localized in the nucleus of primary spermatocytes (Fig. 2D, arrowheads). This nuclear staining of PLD was slightly different from that of 1- or 2-week-old mice. PLD was slightly expressed in Sertoli cells, while PLD was intensely localized in Leydig cells (Fig. 2D, asterisk).
In the mature testis at 8 weeks after birth, intense PLD immunostaining was seen in the primary spermatocytes, with characteristic nuclear localization (Fig. 2E, arrowheads) and in the residual bodies (Fig. 2E, arrows) in the spermatids of the seminiferous tubules. PLD was variably immunostained in spermatogonia in this study. PLD intensely immunostained the Leydig cells in the interstitial spaces (Fig. 2E, asterisk).
This is the first confirmation of the developmental expression of PLD1 and PLD2 in the testis at various time points after birth. The cellular localization of PLD was examined in the testis at various postnatal weeks. Although no immunohistochemical antibody blocking testis using either PLD1 or PLD2 peptides were performed in this experiment, a general pattern of PLD immunoreactivity was identified in various cell types in the mouse testis.
In general, Leydig cells contributes spermatogenesis via the secretion of testosterone, which acts on the Sertoli and/or peritubular cells to create an environment, which enables normal progression of germ cells through stage VII of the spermatogenic cycle [19]. In this study, we have found that Leydig cells constitutively express both PLD1 and PLD2 from postnatal week 1 to 8. This suggests that PLD plays an important role in the biology of Leydig cells, a finding partly consistent with previous studies that PLD was detected in Leydig cells [11,20,21].
In the seminiferous tubules of the testis at weeks 1, 2, 4, and 8 after birth, PLD was expressed in a variety of cells, including undifferentiated cells in the immature tubules (1 and 2 weeks after birth), spermatogonia, primary spermatocytes, and spermatids in the mature tubules (8 weeks after birth). Specifically, we found intense PLD localization in the nuclei of primary spermatocytes, which were ready to divide into secondary spermatocytes and then spermatids. Rather, PLD was intensely immunostained in the residual bodies of spermatids.
Little information has been reported on PLD expression in the Sertoli cells [15]. In this study, we found that most undifferentiated cells in the testis of postnatal 1- to 2-week-old mice were positive for PLD, although the PLD immunoreactivity was very weak. We postulate that only some precursors of Sertoli cells and germ cells express PLD isozymes at this stage. However, we found few PLD-positive Sertoli cells in the testis of 8-week-old mice. Taken together, these findings suggest that PLD is involved in the early postnatal development of the murine testis, but has a very minimal effect in the mature testis, if any.
In summary, PLD is constitutively expressed in the murine testis beginning at 1 week and lasts until 8 weeks postnatally. The cellular localization of PLD implies that PLD is involved in the development of germ cells and endocrine Leydig cells. A further study of the functional role of PLD in the murine testis remains to be performed.
Figures and Tables
Fig. 1
Increased expression of PLD1 and PLD2 protein in the mouse testis at 1, 2, 4, and 8 weeks after birth. Tissue homogenates were immunoprecipitated and immunoblotted with anti-PLD antibody. The data shown are representative of three independent experiments. The anti-PLD antibody specifically recognized PLD1 and PLD2. Molecular size markers are indicated on the left.
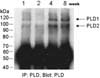
Fig. 2
Immunohistochemical localization of PLD in the testis at 1 (A), 2 (B), 4 (D), and 8 weeks (E) after birth. A and B: PLD immunostained undifferentiated cells in the seminiferous tubules (arrows). Some Leydig cells (asterisks) were immunopositive for PLD. C: Negative control. D: PLD immunostained some primary spermatocytes and intranuclear localization is evident (arrowheads). The asterisk indicates Leydig cells. E: PLD immunoreactivity was seen in some primary spermatocytes (arrowheads) and residual bodies of spermatids (arrows) as well as in Leydig cells (asterisk). Counterstain with hematoxylin. bar = 30 µm.

Acknowledgments
This work was supported by a Program of the Basic Atomic Energy Research Institute (BAERI), which is a part of the Nuclear R&D Programs funded by the Ministry of Science & Technology (MOST) of Korea.
References
1. Ahn M, Min DS, Kang J, Jung K, Shin T. Increased expression of phospholipase D1 in the spinal cords of rats with experimental autoimmune encephalomyelitis. Neurosci Lett. 2001. 316:95–98.

2. Andresen BT, Rizzo MA, Shome K, Romero G. The role of phosphatidic acid in the regulation of the Ras/MEK/Erk signaling cascade. FEBS Lett. 2002. 531:65–68.

3. Baldassare JJ, Jarpe MB, Alferes L, Raben DM. Nuclear translocation of Rho A mediates the mitogen-induced activation of phospholipase D involved in nuclear envelope signal transduction. J Biol Chem. 1997. 272:4911–4914.

4. Boarder MR. A role for phospholipase D in control of mitogenesis. Trends Pharmacol Sci. 1994. 15:57–62.
7. Freyberg Z, Sweeney D, Siddhanta A, Bourgoin S, Frohman M, Shields D. Intracellular localization of phospholipase D1 in mammalian cells. Mol Biol Cell. 2001. 12:943–955.

8. Frohman MA, Morris AJ. Phospholipase D structure and regulation. Chem Phys Lipids. 1999. 98:127–140.

9. Jung K, Min DS, Sim KB, Ahn M, Kim H, Cheong J, Shin T. Upregulation of phospholipase D1 in the spinal cords of rats with clip compression injury. Neurosci Lett. 2003. 336:126–130.

10. Kim H, Lee J, Kim S, Shin MK, Min DS, Shin T. Differential expression of phospholipase D1 and D2 in mouse tissues. Cell Biol Int. 2006. 31:148–155.
11. Lauritzen L, Hansen HS. Differential phospholipid-labeling suggests two subtypes of phospholipase D in rat Leydig cells. Biochem Biophys Res Commun. 1995. 217:747–754.

12. Lee EJ, Min DS, Kang WS, Lee MY, Oh SJ, Chun MH. The expression and cellular localization of phospholipase D1 in the rodent retina. Brain Res. 2001. 905:240–244.

13. Lee EJ, Min DS, Lee MY, Chung JW, Chun MH, Oh SJ. Differential expression of phospholipase D1 in the developing retina. Eur J Neurosci. 2002. 15:1006–1012.

14. Lee MY, Kim SY, Min DS, Choi YS, Shin SL, Chun MH, Lee SB, Kim MS, Jo YH. Upregulation of phospholipase D in astrocytes in response to transient forebrain ischemia. Glia. 2000. 30:311–317.

15. Meier KE, Gibbs TC, Knoepp SM, Ella KM. Expression of phospholipase D isoforms in mammalian cells. Biochim Biophys Acta. 1999. 1439:199–213.

16. Min DS, Ahn BH, Rhie DJ, Yoon SH, Hahn SJ, Kim MS, Jo YH. Expression and regulation of phospholipase D during neuronal differentiation of PC12 cells. Neuropharmacology. 2001. 41:384–391.

17. Min DS, Choi JS, Chun MH, Chung JW, Lee MY. Transient expression of phospholipase D1 in developing rat hippocampus. Neurosci Lett. 2001. 310:125–128.

18. Min DS, Kim EG, Exton JH. Involvement of tyrosine phosphorylation and protein kinase C in the activation of phospholipase D by H2O2 in Swiss 3T3 fibroblasts. J Biol Chem. 1998. 273:29986–29994.

19. Sharpe RM, Maddocks S, Kerr JB. Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am J Anat. 1990. 188:3–20.

20. Strand AM, Lauritzen L, Vinggaard AM, Hansen HS. The subcellular localization of phospholipase D activities in rat Leydig cells. Mol Cell Endocrinol. 1999. 152:99–110.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download