Introduction
Ticks are of great veterinary importance compared to other ectoparasites. They consume large quantities of host blood during their lengthy attachment period (7-14 days), which may be extended depending on the tick species and unique host association.
The bovine tick, Boophilus annulatus (B. annulatus), is a bloodsucking ectoparasite that causes severe production losses in the cattle industry. The average tick burden causes an annual weight loss of 0.7 kg/tick. With the huge number of ticks infesting cattle and other livestock animals, the subsequent effect on beef production is a reduction of hundreds of millions of kilograms annually. Camels, cattle, and chickens are severely affected by ticks, because ticks suck their blood, and may transmit serious pathogenic microorganisms. The control of tick infestations and the transmission of tick-borne diseases remain a challenge for the cattle industry in many areas of the world. Conventional methods used for the control of tick infestation to prevent disease among livestock include the use of chemical toxic acaricides, with partially successful results. However, this treatment has certain implicit drawbacks, such as the presence of residues in the milk and meat, as well as the selection of chemical-resistant tick strains [16,28]. B. microplus has developed resistance to a range of chemical acaricides [1], which has stimulated the development of alternative methods such as vaccination against ticks.
Alternative approaches for tick control, including the use of natural host resistance and development of vaccines to induce an immunological response against tick infestations, have been conducted [21]. A major milestone in vaccine development against the different parasites was the launch of the first genetically-engineered E. coli-expressed Bm86 vaccine which is directed against the cattle tick, B. microplus in Australia [27]. A similar recombinant vaccine which is produced in the Pichia pastoris yeast, was developed and commercialized in Cuba [5,7]. The effect of both vaccines is not a direct killing of ticks, but a successive reduction in numbers as a consequence of a reduction of adult female tick fertility [14].
Development of immunization against the cattle tick, B. annulatus, will present an alternative means by which to control this ectoparasite. The use of several candidate vaccines will reduce the number of acaricide treatments, thus allowing the assumption that savings per animal/year will increase over time. The candidate vaccine will control the transmission of tick-borne diseases such as Babesia bovis infections and anaplasmosis. The major effect of vaccination is not only to kill the ticks currently infecting the herd of cattle, but also to reduce the posterior contamination, and thereby the parasite challenge in the next generation. This vaccine will cause the recovery of bovine erythrocytes, reduce clinical cases, increase milk production, and finally increase meat production.
Several Boophilus tick isolates such as Argentinean strain A showed low susceptibility to vaccination with the well-known commercial vaccines due to some genetic variations [7]. Therefore, research should be continued in order to purify and characterize new immunogenic molecules. In the present study, we report the isolation and purification of two candidate glycoproteins from the larval stage of the cattle tick, B. annulatus. These glycoproteins may be useful for the vaccination of cattle against tick infestation.
Materials and Methods
Ticks and larvae
Adult and nymphal ticks identified as B. annulatus were collected from cattle during several visits to slaughterhouses and field trips to the Matrouh, Gharbiya, and Qalyubia Governorates. Ticks were picked up using pointed forceps. To start different colonies, fully-engorged adults were left to lay egg masses, which were maintained at the animal facility. Ticks were kept under a 14 : 10 light to dark (L : D) photoperiod at 25℃ and 93% relative humidity, according to the procedure described by [17].
Whole tick, larval, and gut antigens
Whole tick and larval antigens of B. annulatus were constructed according to the method of Ghosh et al. [9,10]. In brief, laboratory-reared, clean, 5- to 6-day-old unfed ticks or larvae were homogenized in cold 0.15M phosphate-buffered saline (PBS) and 1mM disodium EDTA, pH 7.2, containing cocktail protease inhibitors, and were then filtered, sonicated, and centrifuged at 15,000 × g for 60 min at 4℃. The supernatant was designated as whole tick or larval antigen. The protein concentrations of the antigens were estimated according to the method of Bradford [3]. Gut antigens were prepared according to the method of Das et al. [6]. In brief, midguts from the partially-fed ticks were dissected out and homogenized in extraction buffer, sonicated, and centrifuged. Supernatants were then collected as gut antigen.
Preparation of rabbit anti-whole tick, larval, and gut antigens
For raising anti-whole tick, larval, and gut antibodies of B. annulatus, three separate male rabbits (≃3 Kg) were immunized by intramuscular injection with 100 µg of whole tick, larval, or gut antigens. The antigens dissolved in 0.5 ml of saline (0.9% NaCl) and mixed with an equal volume of Freund's complete adjuvant (Sigma, USA) were injected on day 0. Rabbits were boosted by 50 µg of the same antigens mixed with Freund's incomplete adjuvant on day 14 by the same route. Seven days after boosting, the rabbits were bled from the marginal ear vein, the serum was pooled, and the immunoglobulins were purified by affinity chromatography using protein G-sepharose CL-4B according to the instructions of the manufacturer.
Immunoaffinity chromatography
The purified immunoglobulins (IgGs) of the rabbit anti-larval antigens were dialyzed against coupling buffer (0.1M NaHCO3, 0.5 M NaCl, pH 8.4) and coupled to Cyanogen bromide-activated Sepharose 4B (CNBr-Sepharose) as recommended by Pharmacia Fine Chemicals (Sweden). The excess reactive sites were blocked by blocking buffer (0.1M glycine-HCl, pH 9.0). The prepared gels were equilibrated with 20 mM phosphate-buffer (PB), pH 7.4, prior to use. Larval antigens of B. annulatus were equilibrated with equilibrating buffer and loaded on the CNBr-Sepharose coupled to the IgGs column at a flow rate of 20 ml/h. The unbound proteins were washed with the equilibration buffer at a flow rate of 30 ml/h. The bound proteins were eluted with 0.1 M glycine-HCl, pH 2.5, into Tris-base in order to restore the pH to 7.2. The eluted proteins were dialyzed against 20 mM Tris-HCl, pH 7.4, and 0.5 M NaCl, and were designated as affinity-purified larval antigen of B. annulatus (aff-LBAg).
Concanavalin A (ConA) affinity chromatography
Glycoproteins (GLPs) were isolated using a ConA-Sepharose column according to the instructions of the manufacturer. The eluted GLPs were equilibrated with 20 mM PB, pH 7.4, concentrated with sucrose, and designated as larval GLPs of B. annulatus. The protein content of the GLPs was estimated by the Bradford method [3].
SDS-PAGE
Electrophoretic analysis was performed in the Mini-Protean II Dual-Slab Cell (BioRad, USA). Preparation of gels, samples, and electrophoresis was performed according to the conditions described by Laemmli [13].
Immunoblotting
Immunoblot analysis was performed using a NovaBlot semi-dry blotter (LKB, Sweden). Preparation of buffers, samples, and the transfer procedure was carried out according to the method of Towbin et al. [24] with slight modifications.
Analytical isoelectric focusing
The electrophoretic analysis was performed in the MultiphorII unit (Pharmacia, Sweden) connected to a thermostatic circulator, and the temperature was set in the range of 4-6℃. Preparation of gels, samples, and electrophoresis was performed according to the method described by Garfin [8] with minor modifications. The gels were stained using the silver stain method according to Rabilloud et al. [19].
Edman degradation
The N-terminal sequences of glycoproteins were determined by automatic Edman degradation using a liquid chromatograph sequencer (Series 1090; Hewlett Packard, USA).
Protein determination
Protein concentrations were determined according to the method of Bradford [3], using bovine serum albumin as standard protein.
Results
Purification of rabbit anti-larval antigens IgGs
The IgGs of rabbit anti-larval antigens were purified using protein G-Sepharose. The rabbit serum was divided into unbound proteins and bound proteins, which were mainly anti-larval IgGs (Fig. 1).
Purification of larval immunogens
The purified IgGs were coupled to Cyanogen bromide-activated Sepharose 4B (CNBr-Sepharose) and used to purify the larval immunogens. Twelve mg of total larval proteins used for immunization were loaded on the CNBr-IgG column. The chromatographic profile of larval antigens on the CNBr-Sepharose coupled to IgGs shows the separation of larval antigens into unbound proteins and bound immunogens (Fig. 2). The total yield of larval bound immunogens was 2.5 mg, which represents around 20.8% of the total larval proteins.
Purification of larval glycoproteins using ConA-Sepharose
The larval immunogens (2.5 mg) were further purified by ConA-Sepharose to purify the larval glycoproteins, which were eluted with 0.2 M methyl α-D glucopyrinoside (Fig. 3). The total amount of larval glycoproteins eluted was 0.625 mg.
Characterization of the larval glycoproteins
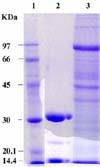
The electrophoretic separation of the larval proteins shows several proteins of high and low molecular weights, while the electrophoretic pattern of the larval glycoproteins shows two major protein bands at molecular weights of approximately 32 and 15 kDa (Fig. 4). The larval antigens and the isolated larval glycoproteins were subjected to an analytical isoelectrofocusing technique using a wide range (3.5-10) of ampholine. The larval antigens were separated into several proteins of different isoelectric points (pI; between 4.5 and 7.0), while the isolated larval glycoproteins were focused between pI 6.8 and 7.2 (Fig. 5).
Immunoblot analysis of the purified larval glycoproteins
Previously prepared antisera separated from the blood samples of rabbit anti-whole tick, whole larval, and gut proteins were used to determine their reactivity with whole larval antigens and the isolated larval GLPs in immunoblots (Fig. 6). The figure shows two protein bands with molecular weights of approximately 32 and 15 kDa, corresponding to the larval GLPs (Fig. 6). The results revealed that the antisera raised against the whole larval, whole tick, and gut antigens contains specific antibodies against these larval GLPs. The reaction of Con-A protein (as a control) with the rabbit anti-larval antigens shows no reactivity of the Con-A protein subunits with this antisera (Fig. 6).
Amino acid sequences of GLPII
The sequence of the first ten residues of the N-terminal amino acid sequences of the 15 kDa GLP was AVDFVTVAVP. This sequence was compared to the protein data bank, and the BLAST analysis revealed 60% and 55% homology with the integral membrane protein 2B from both rat and chicken, respectively (Fig. 7).
Discussion
In concert with the principles of sustainable agriculture, vaccines offer a number of advantages over conventional acaricides/insecticides for the control of arthropod pests. The effect of immunization can be long-lasting and may not include the complications of residues. Vaccines are environmentally-safe, and arthropod resistance to vaccines is less likely to occur than resistance to other treatments [25,26].
The idea of developing immunoprophylactic measures against multi-tick infestations on crossbred animals was based on the concept that ticks feeding on appropriately immunized hosts might ingest antibodies specific for a target antigen(s) within the tick, producing a deleterious effect on their feeding and reproductive performances [2,18]. A few years ago, vaccines containing the recombinant B. microplus gut cell surface antigen, Bm86, were developed [27]. However, B. microplus isolates showing low susceptibility to vaccination with Bm86 appeared [7]. The presence of Bm86-sensitive and -resistant B. microplus strains was thought to result from sequence variations at the B. microplus Bm86 locus. However, sequence variations at the Bm86 locus, among other factors, could affect the effectiveness of Bm86-containing vaccines. All such vaccines on the market target the B. microplus species, which is mainly a problem in Australia and in the Caribbean.
The immunoaffinity chromatographic purification method was previously attempted for the purification of salivary gland antigens of Amblyomma americanum [4], larval antigens [9,10,12], gut origin larval antigen [6], and nymphal antigen [22] of Hyalomma anatolicum. In the case of B. microplus, 3 antigens with molecular weights of 86 kDa [20], 63 kDa [15], and 75-80 kDa [7] were isolated in pure form and tested for their protective potentiality. The presently isolated larval glycoproteins of comparatively lower molecular weights of 32 kDa and 15 kDa may enrich the list of purified tick antigens. These B. annulatus larval glycoproteins will be further characterized, and will be tested for their protective efficacies.
The most important factors on which the success of immunoprotective measure stands are the antigenic dose and its combination with specific adjuvants [23]. Willadsen et al. [29] described two doses of 9.2 and 17.0 µg of 89 kDa glycoprotein per animal in two separate experiments, and used this formulation successfully against B. microplus challenge infestations. The other Australian scientists used 500 µg affinity-purified larval antigens per animal [30]. In the case of H. anatolicum, 2 mg of larval origin 39 kDa protein [9,10], 1.6 mg of gut origin larval antigens [6], and 1.6 mg of nymphal origin of 39 kDa protein [22] were used and found to be protective against homologous challenge.
In the present investigation, a biochemical approach has been adopted to achieve the main goal of vaccine production against B. annulatus. Two specific larval glycoproteins were isolated by two-step affinity chromatography. These glycoproteins have molecular weights of approximately 32 and 15 kDa with isoelectric points between 6.8 and 7.2. These two GLPs reacted with the rabbit antisera against whole tick, whole larval, and gut antigens, which demonstrates that these GLPs have specific antibodies in these antisera. These results suggest that these GLPs may be good immunogens, and can be useful in the vaccination of cattle against cattle tick infestation. The immunogenicity of these proteins could be a result of their positions as membrane proteins. The reaction of these GLPs with the hyperimmune sera from rabbits would confirm the fact that unfed larvae provides an easier source of biological material for the isolation of protective antigens, and is consistent with the data collected by Ghosh et al. [10,11] and Ghosh and Khan [9].
However, the sequence of the first ten residues of the N-terminal amino acid sequence of the 15 kDa GLPII was a small sequence, and the protein-protein BLAST showed a significant similarity with the integral membrane protein 2B from Rattus norvegicus (60%) and Gallus gallus (55%). This integral protein is a glycosylated transmembrane protein with only one potential glycosylation site. More trials will be carried out to complete the sequence of this molecule, which may allow us to determine its structure, modifications, and possible role in the vaccination of cattle against B. annulatus infestations.
These studies may facilitate the use of an mRNA isolation procedure, leading to the application of recombinant DNA technology and in vitro expression of this protein.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download