Abstract
Hematological, cytochemical and ultrastructural features of blood cells in fishing cat (Felis viverrina) were evaluated using complete blood cell counts with routine and cytochemical blood stains, and scanning and transmission electron microscopy. No statistically significant difference was found in different genders of this animal. Unique features of blood cells in this animal were identified in hematological, cytochemical and ultrastructural studies. This study contributes to broaden hematological resources in wildlife animals and provides a guideline for identification of blood cells in the fishing cat.
Fishing cat (Felis viverrina) is dramatically viverrine or civet like appearance. It is the 1 of 9 native wild cats in Thailand [6]. Because of rapid loss of habitats, this wild cat population is declined. Since 2002, the Fishing Cat Breeding Program has developed at Khao Kheow Open Zoo, Thailand. Hematology serves as screening procedure to assess general health and the ability to fight infection and provides information for patient education or diagnosis [3]. However, basic hematological values, cytochemistry and ultrastructures of blood cells have not been described in this species. The purpose of this study was to obtain the hematologic data, cytochemistry and ultrastructures of blood cells in the fishing cat.
Blood were collected from the femoral veins of the 6 male and 7 female clinically healthy fishing cats aged between 2 and 5 years old in Khao Kheow Open Zoo, Thailand. Blood anticoagulated with EDTA was used for hematological evaluation. The completed blood cell count was performed using an automated cell counter (Baker 9110; BioChem ImmunoSystem, USA). Plasma protein was determined by refractometer [4]. Two direct blood smears from each fishing cat were stained with Wright-Giemsa (W-G) and Wright's (W) stains [4]. The diameters of each cell type were randomly measured and at least 200 leukocytes were differentiated. For reticulocytes count, a wet preparation of new methylene blue (NMB) stained smear was applied [4]. The percentage of reticulocytes was determined in 1,000 red blood cells. Means and standard deviations of each hematological parameter and blood cell diameters, were calculated by SPSS for Window version 11.5. Significant difference (p < 0.05) of each parameter between sexes was determined by independent sample Mann-Whitney U-test.
For cytochemical characteristics of each cell type, blood smears were stained with peroxidase (PER) [5], Sudan black B (SBB) [12], β-glucuronidase (β-glu) [2], α-naphthyl acetate esterase (ANAE) [16], and periodic acid Schiff (PAS) [4]. Positive- and negative stained cells were differentiated by counting 500 cells on each stained smears.
For ultrastructural feature of each cell type, blood samples from 4 fishing cats were processed as described elsewhere [9]. Identification was based on the relative number, size, shape, cellular surface, distribution of granules and nuclear appearance.
There was no significant difference of all hematological values between sexes (Table 1). Blood cell diameters were observed and calculated (Table 2). Cytochemical characteristics were summarized (Table 3). The morphology, cytochemistry and ultrastructure of each cell type were evaluated and described below.
Neutrophils were the most prevalent leukocytes (Table 1). They had constricted nucleus with almost indiscernible and faintly pink-bluish stained cytoplasmic granules (Fig. 1A & B). One to three percentages of neutrophils from the females revealed sex chromatin lobe. They were moderately to strongly positive for PER, SBB, β-glu and PAS as the yellow, black, red and magenta granules (Fig. 1C, D, F & G), but weakly positive for ANAE (Fig. 1E). By scanning electron microscope (SEM), they were round with numerous microvilli and micropores (Fig. 7A), but a ruffled membrane protuberance (Fig. 7B) was infrequently observed. By transmission electron microscope (TEM), neutrophils showed lobed nucleus with surface microvilli and numerous cytoplasmic granules (Fig. 7C). The vesicular bodies were found in cytoplasm of some neutrophils.
Eosinophil was 12.5 µm mean in diameter. They presented band or tri-lobe shaped nucleus and numerous cytoplasmic rod-shaped granules (Fig. 2A & B). They were not stained with PER (Fig. 2C) and SBB (Fig. 2D). The refractive granules in red-brown cytoplasm were seen in ANAE staining (Fig. 2E), while reddish periphery of the granules were shown in β-glu (Fig. 2F). With PAS, non-stained granules were presented in strong magenta cytoplasm (Fig. 2G). By SEM, their surface depicted custard-apple like appearance of granule contour with microvilli (Fig. 7D). Ultrastructurally, they were round with lobed nucleus. The cytoplasm was primarily occupied by the characteristic oval to angulated electron dense granules averaged 0.5-1 µm in diameter (Fig. 7E & F).
Basophils were rarely found (Table 1). In W-G stain, they had large lobed nucleus with abundant lavender-stained cytoplasm (Fig. 3A), but a few dark purple granules might be observed with Wright's stain (Fig. 3B). Cytochemically, they were not stained with PER (Fig. 3C) and SBB (Fig. 3D), but revealed light brown when stained with ANAE (Fig. 3E) and bright-red when stained with β-glu (Fig. 3F). They were positive for PAS with diffuse magenta color (Fig. 3G). By SEM, they revealed pleomorphic rod-shaped contour (Fig. 7G). By TEM, basophil granules were prominent and a long-branch projection was found occasionally (Fig. 7H & I).
Monocytes was 13.8 µm in mean diameter (Table 2). The nuclei were extremely variable. They might be round, multilobed or band shape but usually deeply indented with lacy to lopy chromatin. The cytoplasm was moderate to abundant with blue-grey appearance (Fig. 4A & B). Cytochemically, they were negative or slightly positive for PER and SBB as a few small granules scattering in the cytoplasm (Fig. 4C & D). They were stained with ANAE and presented a lot of brown foci (Fig. 4E). They were also positive for β-glu and PAS with diffuse pattern (Fig. 4F & G). By SEM, the wavy appearance with multiple pseudopodia attaching other cells (Fig. 7J) and lamellipodium (Fig. 7K) were frequently observed. By TEM, monocyte was round with deep indented nucleus (Fig. 7L). The cell membrane decorated with indistinct membrane folds and cytoplasmic organelles were easily observed.
The range of lymphocyte sizes were 8.4-15.2 µm in diameters (Table 2). They were divided in 3 sizes; small, medium and large depending on nuclear cytoplasmic ratio. The averaged sizes of small, medium and large lymphocyte were 8.7, 10.9 and 14.5 µm, respectively (Table 2). The cells were round to oval nucleus with or without cleavage appearance and a rim of light blue cytoplasm (Fig. 5A & B). Cytochemically, they were negative for PER and SBB (Fig. 5C & D), but they were positive for ANAE with one or multiple coarse granules in cytoplasm (Fig. 5E). They had 2 patterns of reactivity for β-glu, most were multi-granules (Fig. 5F) and less were single granular stained. They were also positive for PAS with granular pattern (Fig. 5G). By SEM, their surfaces revealed smooth bulgy contour with or without several blebs (Fig. 7M), and a sheet-liked membrane protuberance (Fig. 7N) was occasionally observed. Ultrastructurally, the cell profiles were normally round to oval shape with a few short surface microvilli. The nucleus of small lymphocyte was round to shallow indentation and the cytoplasmic organelles, except mitochondria, were not well presented (Fig. 7O).
Platelets were approximately one-fifth to one-half of red blood cells. They were small round to oval with reddish purple cytoplasmic granules (Fig. 6A & B). Cytochemically, platelets were strongly positive for ANAE (Fig. 6E), weakly positive for β-glu (Fig. 6F) and PAS (Fig. 6G), but were negative for PER and SBB (Fig. 6C & D).
Hematological values and all blood cell morphologies of fishing cats were similar to those of domestic cats [1,8] because they might be the same genus [6]. Anyway, the averaged size of red blood cells in fishing cats (6.3 µm) was larger than that of domestic cats (5.8 µm) [8]. With light and scanning electron microscopes, platelet features were similar to the other mammalian species [3,14]. The indiscernible granules of neutrophils were numerous, small, electron dense when viewed with TEM, while a number of vesicular bodies seen in some neutrophils were considered degranulated granules [13]. By SEM, neutrophils were masked with microvillous projections. These microvilli probably played a significant role in the adhesion to vascular endothelium against the force of blood flow [13], while the blebs or small cytoplasmic projections of lymphocytes unrelated to the functional identity [3].
The most characteristic feature of eosinophil was the prominent eosinophilic granules. They presented large round homogenous electron dense when viewed with TEM. Unlike some other species such as Asiatic black bear [10], Asian elephant [11], domestic cat, dog and horse [13], the crystalline bar-shaped structure in granules were not found. These granules related to the custard-apple like granule contour when viewed with SEM. The granules of basophils were hardly stained with W-G stain. Only few dark granules were seen in Wright stained smear, but they were clearly seen by TEM. The lamellipodia seen by SEM, could be largely extended either on cell margin or cell center, and was seen commonly on monocytes and occasionally on some lymphocytes. These findings relate to the behavior of the cells, especially the monocytes were able to become macrophages which often extended processes such as pseudopodia [13].
Although cytochemical patterns of blood cells were different among the various species, but the cytochemical patterns of blood cells in this study were similar to those of domestic cats [15]. The reactivity of PAS was found within a wide variety of cells, including granulocytes, agranulocytes and platelets referred to the storage of glycogen [7]. Monocytes and lymphocytes exhibited unique pattern of ANAE reactivity. A brown, diffusely granular pattern was seen in monocytes, while a single focal or punctuate reaction product was seen in lymphocytes. The different patterns related to the location of the enzyme in these cells. ANAE had been identified mostly on the plasma membrane of monocytes, but only within intracellular organelles of lymphocytes [7].
In summary, we have found that blood cell morphologies of fishing cat were similar to those of domestic cat, especially rod shaped granules of eosinophil and lavender stained cytoplasm of basophil. Cytochemistry provided the staining patterns among the cell types. Electron microscope was beneficial in the identification of cellular surfaces as well as cytoplasmic granules and organelles. This information provides a guideline for identification of blood cells in the fishing cat.
Figures and Tables
Fig. 1 to 6
Romanowsky's and selected cytochemical staining reactions for normal blood cells in fishing cat.
Fig. 1. Neutrophils: (A & B) a constricted nucleus with indiscernible cytoplasmic granules, one Howell-Jolly bodies nearby (arrow),
WG and W, respectively. (C & D) strongly positive neutrophils, PER and SBB, respectively. (E) weakly positive for ANAE. (F)
positively stained for β-glu. (G) strongly positive for PAS.
Fig. 2. Eosinophils: (A & B) an eosinophil with rod-shape granules, WG and W, respectively. (C & D) refractive granules in non stained cytoplasm, PER and SBB, respectively. (E) refractive granules in red-brown cytoplasm, ANAE. (F) positive in the periphery of the granules, β-glu. (G) non-stained granules in strong magenta cytoplasm, PAS.
Fig. 3. Basophils: (A) a basophil with large lobed nucleus and lavender-stained cytoplasm with WG. (B) a few dark purple granules in cytoplasm with W. (C) negative for PER. (D) a negatively stained basophil (right) compared to a positively stained neutrophil (left), SBB. (E & F) positive for ANAE and β-glu, respectively. (G) positive with fine granular pattern of basophil (right) compared to positive with diffuse pattern of neutrophil (left), PAS.
Fig. 4. Monocytes: (A & B) a monocyte with deep indented nucleus, WG and W, respectively. (C) slightly positive for PER. (D) slightly positive with a few black granules of monocyte (left) compared to strongly positive neutrophil (right), SBB. (E, F & G) positive for ANAE, β-glu and PAS, respectively.
Fig. 5. Lymphocytes: (A & B) a lymphocyte with condense nuclear chromatin and thin band cytoplasm, WG and W, respectively. (C & D) negative for PER and SBB, respectively. (E, F & G) positive for ANAE, β-glu and PAS, respectively.
Fig. 6. Platelets: (A & B) group of small round, anucleated cells with reddish purple granules, WG and W, respectively. (C & D) negative for PER and SBB, respectively. (E) strongly positive for ANAE. (F & G) weakly positive for β-glu and PAS, respectively.
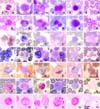
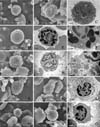
Fig. 7
Cellular surfaces and ultrastructures of various white blood cells in fishing cat. (A) a neutrophil showing numerous microvilli on its surface in contact with one platelet, SEM. (B) a neutrophil with ruffled membrane protuberance, SEM. (C) a neutrophil presenting short microvilli, lobulated nucleus, cytoplasmic granules and vesicular bodies, TEM. (D) an eosinophil showing custard-apple liked appearance of granule contour with microvilli, SEM. (E) an eosinophil containing characteristic large granules, TEM. (F) higher magnification of (E), presenting round to angulated electron dense granules ranging 0.5-1 µm in diameter. (G) a basophil revealed irregular rod shaped granule contour, SEM. (H) a basophil showing large lobed nucleus with heterogenous granules, membrane folds, microvilli and irregular branch projection, TEM. (I) higher magnification of (H) showing basophil granules. (J) a monocyte showing wavy appearance with pseudopodic projections, SEM. (K) a monocyte showing lamellipodium extended from the cell center, SEM. (L) a monocyte showing deep indented nucleus and less electron dense cytoplasm, TEM. (M) a lymphocyte presenting bulgy contour with several blebs, SEM. (N) a lymphocyte showing lamellipodium extended from cell margin, SEM. (O) a lymphocyte revealed nuclear indentation with cell membrane folds and a few microvillous projections, TEM.
Acknowledgments
This research was supported in part by Grant from the Faculty of Graduate Studies, Mahidol University, Thailand (2005).
References
1. Clinkenbeard KD, Meinkoth JH. Feldman BF, Zinkl JG, Jain NC, editors. Normal hematology of the cat. Schalm's Veterinary Hematology. 2000. 5th ed. Philadelphia: Lippincott Williams & Wilkins;1064–1068.
2. Hayhoe FGJ, Quaglino D. Haematological Cytochemistry. 1980. Edinburgh: Churchill Livingstone;68–75.
3. Jain NC. Essentials of Veterinary Hematology. 1993. Philadelphia: Lea & Febiger;417.
4. Jain NC. Schalm's Veterinary Hematology. 1986. 4th ed. Philadelphia: Lea & Febiger;1221.
5. Kaplow LS. Simplified myeloperoxidase stain using benzidine dihydrochloride. Blood. 1965. 26:215–219.
6. Lekagul B, McNeely JA, editors. Family felidae. Mammals of Thailand. 1988. 2nd ed. Bangkok: Darnsutha Press;603–630.
7. Raskin RE, Valenciano A. Feldman BF, Zinkl JG, Jain NC, editors. Cytochemistry of normal leukocytes. Schalm's Veterinary Hematology. 2000. 5th ed. Philadelphia: Lippincott Williams & Wilkins;337–346.
8. Reagan WJ, Sanders TG, DeNicola DB. Veterinary Hematology: Atlas of Common Domestic Species. 1998. Ames: Manson Publishing;75.
9. Salakij C, Salakij J, Apibal S, Narkkong NA, Chanhome L, Rochanapat N. Hematology, morphology, cytochemical staining, and ultrastructural characteristics of blood cells in king cobras (Ophiophagus hannah). Vet Clin Pathol. 2002. 31:116–126.

10. Salakij C, Salakij J, Narkkong NA, Trongwonsa L, Pattanarangsan R. Hematology, cytochemistry and ultrastructure of blood cells from Asiatic black bear (Ursus thibetanus). Kasetsart J (Nat Sci). 2005. 39:247–261.
11. Salakij J, Salakij C, Narkkong NA, Apibal S, Suthunmapinuntra P, Rattanakunuprakarn J, Nunklang G, Yindee M. Hematology, cytochemistry and ultrastructure of blood cells from Asian elephant (Elephas maximus). Kasetsart J (Nat Sci). 2005. 39:482–493.
12. Sheehan HL, Storey GW. An improved method of staining leukocyte granules with Sudan black B. J Pathol Bacteriol. 1947. 59:336–337.
13. Steffens WL. Feldman BF, Zinkl JG, Jain NC, editors. Ultrastructure features of leukocytes. Schalm's Veterinary Hematology. 2000. 5th ed. Philadelphia: Lippincott Williams & Wilkins;326–336.
14. Tablin F. Feldman BF, Zinkl JG, Jain NC, editors. Platelet Structure and Function. Schalm's Veterinary Hematology. 2000. 5th ed. Philadelphia: Lippincott Williams & Wilkins;448–452.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download