Introduction
Microsporidia are intracellular parasites that infect many animal groups, including freshwater cultured fish [10,13]. Microsporidia, which infect sarcocytes of the trunk muscle of fish, are represented by the genera Pleistophora Gurley 1893, Heterosporis Schubert 1969 and Kabatana Lom, Dyková and Tonguthai 2000 [7,8,9,11]. Infections in species of the genus Heterosporis may also have significant pathogenic effects on their hosts. This species does not produce a xenoma but infects the tissues diffusely and might eventually be surrounded by host connective tissue [6]. Of particular economic significance are those species infecting the muscle tissue because infected host cells produce grossly visible lesions that render the flesh unfit for human consumption. The key feature of the genus Heterosporis on electron microscopy images is the presence of a sporophorocyst (SPC), which is a dense, rather solid wall enclosing all the developmental stages of the parasite, i. e., meronts, sporonts and sporophorous vesicles with sporoblasts and spores [8]. The species deserves particular attention because it has the pathogenic potential to endanger the cultured Southeast Asian eel and is a possible threat to cultured eels worldwide [6,8,12]. In view of the economic importance of the species, there are several reports on this species [2,4,5]. Heterosporis infections have been suspected to be endemic in cultured eel farms in South Korea, with significant economical losses [12]. This disease is caused by an infection from Heterosporis anguillarum in the musculature, which lyses the muscles, giving an uneven appearance of the trunk of infected eels. This paper reports the occurrence of the Heterosporis infections in February 2004 as well as the pathological features and transmission electron microscopy (TEM) images of the Heterosporis anguillarum found in infected eels in Jeonbuk Province, Korea.
Materials and Methods
Fish samples
Young eels were collected in the wild last year before spring, reared and wintered. The uneven external appearance was first noticed in 5 cages on 2nd Feb. 2004. For ten days, the number of eels affected increased to approximately 2,000, which manifested as an unevenness of the trunk.
Examination of the clinical sings and gross lesions
To investigate the Heterosporis infection, the eels showing clinical signs were transferred from nursery ponds to the laboratory and examined for any external and internal lesions. In the postmortem necropsy, the existence of parasites, particularly microsporidia, was carefully examined from trunk part of the body containing the epidermis, dermis, and musculature.
Examination of histopathological and electron microscopy
For the histopathology, muscle tissues and internal organs of the samples were fixed by submersion in 10% neutral buffered formalin, dehydrated through a graded series of ethanol, and embedded in paraffin. Five-micrometer thick sections were made and stained with hematoxylin and eosin.
For TEM, a part of the formalin-fixed tissues was washed in running tap water, post-fixed for 2 h in 1% osmium tetroxide in PBS at 4℃, washed again in the same buffer for 5 h, and then dehydrated through a graded series of ethanol and critical-point dried. Before infiltration with an epon mixture, the samples were exchanged once with propylene oxide for 30 min. After double staining with uranyl acetate and lead citrate, the materials were examined by TEM (H-7100FA; Hitachi, Japan) at an acceleration voltage of 75 kV.
Results
Gross pathological findings
The most common lesions observed in the infected eels were unevenness of the trunk with the skin appearing somewhat pale. The unevenness of the trunk appeared to show turns within normal appearing skin and sunken parts. The normal parts were sound and solid but the sunken parts appeared pasty. The necropsies revealed the musculature in the sunken parts to be severely damaged, and the muscle tissue had been lysed and converted to a whitish material.
Histopathology findings
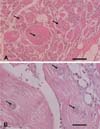
Optical microscopy of the lysed muscle tissues revealed degenerative muscle fibers, scattered sporophorocyst and spores around the muscles (Fig. 1). The SPC contained spores with a variety of shapes. Moreover, some SPC were disrupted and the spores in the SPC were scattered through the muscle tissues. The macrophages existed near the scattered spores (Fig. 1).
Electron microscopy
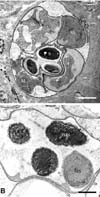
The SPCs were observed by TEM to be bounded by a dense envelope in the muscle tissues of the eels (Fig. 2). The SPCs contained various developmental stages of the parasite containing sporophorous vesicles with spores, sporonts and meronts (Fig. 2A). The immature spores contained 2 large dense globules (about 1.5 to 2 µm in size) in the space of a future posterior vacuole. The mature spores contained a large posterior vacuole filled with dense floccular material and encircled by turns of a coiled polar tube (Fig. 2B).
Discussion
In microsporidia, although there are 14 genera, only Heterosoridis anguillarum was identified in the clinical lesions, infection sites and spores by TEM. The size and form of the spore were the same as those previously reported [1,2,8]. Lom and Nilsen [8] divided fish microsporidia into 5 groups by molecular phylogeny. Group 1 contained Pleistophoridae Doflein, which consisted of the following 5 genera: Pleistophora, Heterosporis, Ovipleistophora, Vavraia and Trachipleistophora. Three of them infect fish, and Heterosporis is known to infect the muscle tissue of fish. The morphology of Heterosporis anguillarum differs from other species from the genus Pleistophora [6,8]. They reported 2 significant differences; the presence of the SPC and the absence of small canals leading through the massive sporophorous vesicle. The development of spores in Heterosporis anguillarum is dependent on the water temperature. When the temperature is between 15-30℃, spores begin to develop, causing the disease. When the temperature is >25℃, disease appears earlier and gross lesions can be detected within 20 days from an experimental infection [12].
In Korea, most farms rear eels in a heating-house system to maintain a water temperature between 25-30℃. Once the eels are infected with the Heterosporis anguillarum, the possibility of transmission is high. Therefore, the prevention of Heterosporis anguillarum infections is more important than to trying to cure the disease. It is believed that first way of preventing an occurrence of the parasitic disease is to prevent the flow of unfiltered underground water or contaminated water into a culture farm from the field.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download