Abstract
A total of 75 biopsied samples of cardia, fundus, body, and pyloric antrum from necropsied dogs that were submitted to the Department of Pathology, Faculty of Veterinary Science, Chulalongkorn University from April 2003 to June 2004 were investigated. The objectives of this study were to determine the prevalence of Helicobacter spp. in canine stomach by polymerase chain reaction (PCR) in comparison to histochemistry versus immunohistochemistry (IHC), and to correlate these diagnostic methods with the clinical significance in infected dogs. Histopathological results revealed 60.0% (45/75) of samples to be positive, and consisted of mild gastritis in 64.44% (29/45), moderate gastritis in 11.11% (5/45), and severe gastritis in 24.44% (11/45). The proportion showing no histopathological lesions was 40.0% (30/75). Helicobacter spp. were localized to the luminal crypt in 18.67% (14/75), gastric pit in 22.67% (17/75), gastric gland in 21.33% (16/75), and gastric epithelium in 8% (6/75). The percentages of positive samples of Helicobacter spp. diagnosed by hematoxylin and eosin stain (H&E), Warthin Starry stain (WSS), IHC with rabbit polyclonal anti-H. pylori antibody, and PCR were 17.3% (13/75), 46.7% (35/75), 30.7% (23/75), and 10.7% (8/75), respectively. No significant differences weree observed in histopathological changes in portions of the stomach (p>0.05). The diagnosis of Helicobacter spp. by PCR in comparison to that by WSS and IHC was not significantly different (p>0.05). There were no relationships between pathological studies using H&E, WSS, and IHC, and especially between PCR and clinical signs of Helicobacter spp. infections in canine stomachs (p>0.05). The present study revealed significantly different levels of correlation for Helicobacter spp. detection between H&E and WSS (p<0.001). Results indicate that the method of choice for diagnosis of Helicobacter spp. infection in canine stomach is dependent on the purpose of study and appropriate specimen collection.
A gastric bacteria categorized in genus Helicobacter spp. are Gram-negative and spiral-shaped. At least 24 species have been formally reported, and most Helicobacter spp. are suspected or proven gastric or hepatic pathogens in humans [9,12]. Investigation of the relationship between gastric disease and Helicobacter spp. infection in other species has resulted in the discoveries of H. mustelaein ferrets with gastritis and peptic ulcers, H. acinonyx in cheetahs with severe gastritis, and H. heilmannii in pigs with gastric ulcers [5,10,25].
The role of Helicobacter spp. in gastrointestinal disease in dogs and cats is uncertain. It has been known for years that gastric Helicobacter-like organisms (HLO) are commonly present in the stomachs of dogs, but the relationship between these organisms and gastric disease has never been resolved [6,12,15,16,18,29]. A study of canine gastric Helicobacter spp. infection was performed in Thailand. This study revealed significantly different correlations in terms of bacterial detection between hematoxylin and eosin stain (H&E) and Warthin Starry stain (WSS) [23]. Infection with HLO is highly prevalent in dogs, and Helicobacter spp. has been found in 61-80% of dogs presenting with vomiting and in 67-86% of clinically healthy pet dogs [6,12,18,29].
Diagnostic tests for Helicobacter spp. can be divided into two methods, invasive and noninvasive. A practical Helicobacter pylori (H. pylori) ELISA assay has been developed and used [17]. Direct observation of Helicobacter spp. organisms in biopsied specimens usually requires special stains, e.g. Giemsa, WSS, Genta, and alcian yellow-toluidine blue stain. Typically, H. pylori is not visualized by H&E staining [28]. Current standards in human medicine dictate that an estimate of H. pylori density, activity and grade of gastritis, and comments on the presence of atrophic gastritis or intestinal metaplasia should be provided when histopathological examination is conducted [20]. The specificity of histopathology is 100%, and sensitivity is greater than 90% [22,28]. Immunohistochemical staining and electron microscopy may also be used to evaluate gastric biopsy specimens [17,28]. Polymerase chain reaction (PCR) allows the identification of the particular species or strain of Helicobacter, and samples used for PCR can be gastric biopsies, gastric juice, dental plaque, or feces. The sensitivity of the detection method, varied according to the primer used, but the sensitivity was considerably high [17,22,28]. In addition, PCR allows identification of specific H. pylori genes (so-called virulence factors) associated with an increased incidence of peptic ulcer or cancer in humans [17].
The objectives of this study were to determine the prevalence of Helicobacter spp. in canine stomach using PCR in comparison to histochemistry versus immunohistochemistry (IHC), and to correlate these diagnostic methods with the clinical significance in infected dogs. The results from this experiment could be useful in elucidating the epidemiology and diagnosis of Helicobacter spp. in dogs.
Gastric tissues were randomly collected from 75 necropsied dogs submitted to the Department of Pathology, Faculty of Veterinary Science, Chulalongkorn University. Their detailed descriptions were recorded as in routine necropsy cases, and included information such as breed, sex, age, clinical signs, and cause of illness or death. The stomach of the dog was then opened along the greater curvature and inspected for gross lesions. Two pieces of gastric mucosa tissue were then divided into four different stomach sections; cardia, fundus, body, and pyloric antrum. The first half of each gastric section was then placed in a sterilized Eppendorf tube containing a 0.9% sterile NaCl solution and frozen at -70℃ for subsequent DNA extraction and PCR analysis. The remaining half was placed in 10% buffered formalin (pH 7) for histopathology [23].
The formalin-fixed tissues were trimmed and processed in a tissue processor using a routine histopathological method. In brief, tissues were dehydrated by 70, 80, 95, and 100% ethanol and xylene, respectively. Tissues were embedded in a paraffin block and cut into 4-6 µm thickness by microtome, and sections were then placed on slides (for H&E and WSS) or poly-L-lysine-coated slides (for IHC). The paraffin-embedded sections were deparaffinized and rehydrated by xylene, 100, 95, 85, and 70% ethanol and distilled water, respectively. The slides were stained with Harris hematoxylin and then dehydrated by 70, 80, 95, and 100% ethanol and xylene, respectively.
The H&E-stained slides, which were evaluated on a blindcoded basis, were assigned gastritis scores according to the following criteria: 0, normal, containing 0 to 10 inflammatory cells (not including those within lymphoid aggregates) per high-power field (×400) with no lymphoid follicular aggregates and normal mucosal epithelium; 1, mild gastritis, containing 10 to 50 inflammatory cells per high-power field with fewer than 2 follicles per low-power field (×20) and normal mucosal epithelium; 2, moderated gastritis, containing 10 to 50 or more inflammatory cells per high-power field with greater than two follicles per low-power field and mild gastric epithelial changes; 3, severe gastritis, containing greater than 50 inflammatory cells per high-power field and marked epithelial changes. Epithelial changes included individual cell necrosis, basophilic cytoplasm, and glandular dilation. The type and location of inflammatory cells and the number of lymphoid follicles were noted, as were spiral organisms. Helicobacter spp. showed basophilic color and a spiral shape, and were 2.5-5.0 µm long and 0.5-1.0 µm wide [14,23]. WSS stained sections were stained as previously described [21]. The slides were evaluated for the presence of organisms and the morphologies of the organisms on a blind-coded basis. The enumeration of organisms seen in each 1.5 cm stained tissue section was graded according to the following scale: 0, no organisms seen; 1, few organisms (<10 organisms per section); 2, moderate numbers of organisms (10 to 50 organisms per section); 3, large numbers of organisms (>50 organisms per section, usually too numerous to count) [14,23]. Spiral-shaped Helicobacter spp. was considered to be positive if it appeared dark brown on a yellow background, and was 2.5-5.0 µm long and 0.5-1.0 µm wide.
IHC of the consecutive sections was also employed for immunohistochemical analyses using the ABC-peroxidase technique with a rabbit polyclonal anti-H. pylori antibody (DAKO, Denmark). Evaluation was rated as follows: negative, no Helicobacter spp.; positive, the presence of Helicobacter spp. antigen, indicated with a brown color [7,23].
DNA extraction: From each gastric tissue sample, the mucosa was scraped from tissue using a surgical blade. DNA was isolated from 25 mg of the scraped tissue, which was extracted by the QIAmp Tissue Kit (Qiagen, USA) used according to the instructions of the manufacturer.
PCR primers: A pair of primers was used for PCR (Table 1). Primers H 1 and H 2 specific to the Helicobacter genus were prepared on the basis of the 16S rRNA gene sequences of the Helicobacter-specific sequences. The H276f/H676r primer pair was selected on the basis of alignments performed using the EuGene software package (Baylor College of Medicine, USA) [26] as described previously [2,3].
PCR amplification: All reactions were performed in a 25 µl volume with an automated thermocycler (HBPxE 0.2; Thermo Hybaid, UK) at the Veterinary Diagnostic Laboratory, Chulalongkorn University. Reaction mixtures contained each oligonucleotide primer at 5 µM, PCR buffer (10 mM Tris-HCl, 2 mM MgCl2, 1 mM dNTP), 1 U of Hotstat Taq polymerase (MBI Fermentas, USA), 6 µl of template DNA, and distilled water in a total volume of 25 µl. The initial dehydration step samples were heated at 95℃ for 5 min, followed by the first denaturation step (94℃, 1 min) at 35 cycles, a primer annealing step (53℃, 1 min), an extension step (72℃, 1 min), and a final extension step (72℃, 5 min). The PCR products (10 µl) were subjected to electrophoretic separation in a 2% agarose gel (Fisher Chemical, USA) by electrophoresis at 100 V, 1.5 A for 1 h, stained with ethidium bromide (Promega, USA) for 20 min, and then washed with distilled water. The DNA band was analyzed in the UV illuminator. H. pylori from human, used as a positive control, was kindly obtained from the Department of Microbiology, Chulalongkorn Memorial Hospital, and nuclease-free water was used as a negative control.
The Chi-square test was used to examine the relationship between the location of histopathological lesions and Helicobacter spp. infection. Chi-square analysis was used to investigate the relationships between pathological studies using H&E, WSS, and IHC. Fisher's exact test was used to determine the relationship between pathological studies by H&E and PCR of H. pylori infections in canine stomachs. The Wilcoxon rank sums test was used to examine the relationship between pathological studies using WSS and PCR. A p-value <0.05 was considered to be significant. All statistical analyses were performed using SAS software (SAS Institute, USA).
The clinical symptoms in 75 necropsied dogs were reported as loss of appetite in 32% (24/75), vomiting in 22.7% (17/75), and diarrhea in 6.7% (5/75). Macroscopic lesions were reported in 34.7% (26/75), and can be further broken down as focal ulcerative gastritis, 10.7% (8/75); catarrhal gastritis, 8% (6/75); hemorrhagic gastritis, 13.3% (10/75); erosive gastritis, 1.3% (1/75); and gastric polyps, 1.3% (1/75) (Fig. 1).
Microscopic lesions such as lymphoid follicular formation in submucosa and inflammatory cell infiltration in mucosa were observed in our samples. Most of the neutrophil infiltration in ulcerative gastritis, crypt distortion, and epithelial degeneration in the study location of the stomach were found in cardia, 34.7% (26/75); fundus, 30.7% (23/75); body, 30.7% (23/75); and pylorus, 21.3% (16/75). The histopathological results revealed lymphoid follicular formation in 33.3% (25/75); inflammatory cell infiltration, 25.3% (19/75); epithelial degeneration, 17.3% (13/75); and crypt distortion, 13.3% (10/75) (Fig. 2). Histopathological lesions of the stomach were observed in 60.0% (45/75); mild gastritis in 64.4% (29/45), moderate gastritis in 11.1% (5/45), and severe gastritis in 24.4% (11/45) (Table 2).
Using H&E staining, the presence of Helicobacter spp. was observed in 17.3% of samples (13/75). Using H&E, Helicobacter spp. was detected in 10.7% of cardia samples (8/75), 6.7% of fundus samples (5/75), 12% of body samples (9/75), and 8% of pylorus samples (6/75) (Fig. 3). Helicobacter spp. was observed in large numbers in the mucus covering the surface gastric epithelium, the gastric pits, and the glandular lumina.
The histopathological evaluation of WSS sections revealed the presence of spiral-shaped organisms measuring approximately 3 to 5 µm in length and 0.5 µm in width in 46.7% of samples (35/75). Using WSS, Helicobacter spp. was detected in 25.3% of cardia samples (19/75), 21.3% of fundus samples (16/75), 30.7% of body samples (23/75), and 20% of pylorus samples (15/75) (Fig. 4).
The IHC staining using rabbit polyclonal anti-H. pylori antibody revealed a brown color at positive sites in 30.67% of tissue samples (23/75). The presence of Helicobacter spp. antigen in cardia was 16.0% (12/75), and the presences detected in the fundus, body, and pylorus were 13.3% (10/75), 10.7% (8/75), and 12.0% (9/75), respectively (Fig. 5).
The correlations between the detection methods are shown in Table 4. The present study revealed a statistically significant difference in Helicobacter spp. detection between H&E and WSS (p < 0.001), as well as a correlation between H&E and IHC (p < 0.05), and H&E and PCR (p < 0.05). Histopathological results were found to be related to WSS in Helicobacter spp. infections (p < 0.05). The pathological studies conducted using H&E, WSS, IHC, and PCR and clinical signs of Helicobacter spp. infections in canine stomachs were not found to be statistically different (p > 0.05). There was no statistically significantly different relationship was observed between IHC and PCR, nor was there a significant correlation between portions of histopathological lesions and Helicobacter spp. infection (p > 0.05) (Table 5). The numbers of Helicobacter spp. isolates detected by histopathological methods did not differ significantly across each part of the stomach (p > 0.05).
The results of this study emphasize other studies, which frequently suggested the presence of Helicobacter spp. in dogs, with a prevalence of approximately 46.7% [12,16,18,23]. It has been reported that 82% of canine gastric biopsies were infected with Helicobacter-like organisms, and it was postulated that they were infected with several species of Helicobacter spp. [18]. The most frequent clinical symptoms are loss of appetite, vomiting, and diarrhea [18,19], which is in agreement with the necropsies in this study at 52% (39/75). However, several recent reports have indicated that chronic gastritis, which is associated with intermittent vomiting, is also observed in dogs, but the etiology of this condition is rarely diagnosed [8]. A statistically significant correlation between Helicobacter spp. infection and clinical symptoms could not be established (p > 0.05).
Gastric Helicobacter spp. was found in the cardia, fundus, body, and pylorus [18]. In this study, all parts of the stomach had Helicobacter spp.; 34.7% of cardia (26/75), 30.7% of fundus (23/75), 30.7% of body (23/75), and 21.3% of pylorus samples (16/75), and these results are similar to those reported by Pirarat et al. [23]. No statistically different significant correlation was detected between the locations of histopathological lesions and Helicobacter spp. infection using the H&E detection method (p > 0.05). The presence of lymphoid follicles has traditionally been a common, nonspecific finding in the gastric mucosa of dogs. Most dogs had many bacteria in the stomach, but only developed mild gastritis. This suggests that in dogs, certain bacterial diseases do not show histological evidence [5,27]. This study revealed significantly different correlations of Helicobacter spp. detection between H&E and WSS across parts of the stomach (p < 0.001). Helicobacter spp. can be visualized at high magnification in conventional H&E stained sections. Bacteria are located in the mucus adherent to the surface epithelium, and are often found deep within the crypts. Helicobacter spp. could be detected by H&E in 10.7% of cardia, 10.7% of body, 6.7% of fundus, and 6.7% of pylorus samples. However, H&E staining may have low sensitivity when few bacteria are present [4], so the use of special stains such as WSS facilitates histological identification of bacteria. Helicobacter spp. was obviously presented in a dark-brown color on a yellow background. WSS sections of portions of the stomach revealed tightly coiled helical micro-organisms that were generally 2.5-5.0 µm long. According to the results of this study, Helicobacter spp. was found in 30.7% of body (23/75), 26.7% of cardia (20/75), 21.3% of fundus (16/75), and 20.0% of pylorus samples (15/75). Therefore, in terms of histological detection, WSS is significantly more sensitive than H&E [4].
The present study revealed a significant difference in Helicobacter spp. detection between H&E and IHC (p < 0.05). IHC was much more sensitive for detecting infection than the routinely used H&E and WSS. IHC staining has also been developed to detect Helicobacter spp. antigen [1]. In this study, the ABC-peroxidase technique with a rabbit polyclonal anti-H. pylori antibody was used. IHC using monoclonal anti-H. pylori antibody is the best diagnostic tool for formalin-fixed samples. Although IHC is highly specific [7], IHC is rather expensive and requires both time and experience. Therefore, IHC usually is not used for routine testing, but may prove worthwhile in cases where conventional histological stains are difficult to evaluate for confirmation [4].
This study revealed significantly different correlations in Helicobacter spp. detection between H&E and PCR (p < 0.05). PCR offers great promise as a highly sensitive and specific technique for the detection of Helicobacter spp. The identification of Helicobacter spp. in biopsies from dogs using PCR has not been reported previously, but has been found to be especially useful for the detection or the identification of Helicobacter spp. in dogs with naturally occurring gastric Helicobacteriosis in Thailand. Several observations have shown that PCR was sensitive and specific, which is in agreement with studies in mice infected with H. felis and in humans and cats infected H. pylori, which showed that PCR was more sensitive than histology, bacterial culture, and urease mapping [27]. Otherwise, in this study, Helicobacter spp. were found in 10.67% of samples (8/75) using PCR. Like other clinical symptom comparisons, no relationship was detected between PCR and clinical symptoms of H. pylori infection in canine stomach (p > 0.05).
The prevalence of Helicobacter spp. was low in this study using the PCR technique, because possible factors such as sampling location, specimen preparation, the choice of primers and target DNA, bacterial density and technical error, the DNA extraction step, or inhibitors of gastric juice and tissue may affect PCR detection [4]. In this study, canine gastric tissues were randomly collected from each part of the stomach with and without lesions. Primers and target DNA were selected based on an earlier study. A 400 bp fragment was clearly seen by 2% agarose gel electrophoresis following PCR amplification of the positive control (106 CFU/µl H. pylori from human). However, the method by which DNA is extracted from the tissue samples should be discussed, and may have disturbed the amplification of the fragments of DNA because of some blocking factor [24]. The Helicobacter spp. detected by PCR may have been H. heilmannii, H. felis, or H. bizzozeronii [8,10,14].
In future study, fresh canine gastric biopsies might be suitable specimens for use in the study of Helicobacter spp. infections in terms of bacterial isolation and PCR methods, as well as other invasive methods, and more species-specific primers should be employed. Furthermore, the DNA sequencing of PCR products for comparison between Helicobacter spp. found in dogs and their owners should be beneficial in terms of veterinary public health. The present results will be useful as a diagnostic tool for monitoring species causing Helicobacter spp. infection in dogs.
Figures and Tables
Fig. 1
Macroscopic lesions were shown as chronic mutifocal ulcerative gastritis; a 0.5 × 1.5 cm ulcer was shown on the gastric mucosa in the body part of the stomach (green arrowhead).
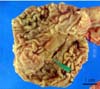
Fig. 2
The histopathological results revealed the formation of multiple lymphoid follicles, which were numerous in gastric mucosa. H&E stain.
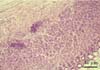
Fig. 3
The numerous spiral-shaped organisms: Helicobacter spp. is shown in the surface of gastric mucosa in the fundus of the stomach. H&E stain.
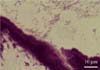
Fig. 4
Spiral-shaped Helicobacter spp. was positive, as indicated by a dark brown color, and was 3.0-5.0 µm in length and 0.5-1.0 µm in width in the gastric pit of the fundus. At a higher magnification, Helicobacter spp. with a spiral shape and dark brown color are shown. Warthin Starry stain.

Fig. 5
Helicobacter spp. showed a brown color in positive sites by immunohistochemical staining with rabbit polyclonal anti-H. pylori antibody in the lumen of the gastric gland in the fundus portion of the stomach. Counterstaining with Meyer's hematoxylin.
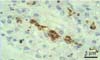
Fig. 6
The 400 bp bands were detected by 2% agarose gel electrophoresis following PCR amplification of the Helicobacter spp.-specific sequences. Lane 1-8: positive results from canine gastric tissues; Lane 9: positive control H. Pylori from human; Lane 10: negative control nuclease-free water; Lane 11: 100 bp DNA marker ladder.
Acknowledgments
The authors would like to express their sincere thanks to Research Funding, Faculty of Veterinary Science and Graduate School, Chulalongkorn University academic year 2004-2005.
References
1. Ashton-Key M, Diss TC, Isaacson PG. Detection of Helicobacter pylori in gastric biopsy and resection specimens. J Clin Pathol. 1996. 49:107–111.

2. Buczolits S, Hirt R, Rosengarten R, Busse HJ. PCR-based genetic evidence for occurrence of Helicobacter pylori and novel Helicobacter species in the canine gastric mucosa. Vet Microbiol. 2003. 95:259–270.

3. Buczolits S, Rosengarten R, Hirt R, Busse HJ. Classification of a Brevundimonas strain detectable after PCR with a Helicobacter-specific primer pair. Syst Appl Microbiol. 2001. 24:368–376.

5. Eaton KA, Radin MJ, Kramer L, Wack R, Sherding R, Krakowka S, Fox JG, Morgan DR. Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus). Vet Pathol. 1993. 30:55–63.

6. Eaton KA, Dewhirst FE, Paster BJ, Tzellas N, Coleman BE, Paola J, Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996. 34:3165–3170.

7. Esteves MI, Schrenzel MD, Marini RP, Taylor NS, Xu S, Hagen S, Feng Y, Shen Z, Fox JG. Helicobacter pylori gastritis in cats with long-term natural infection as a model of human disease. Am J Pathol. 2000. 156:709–721.

8. Flatland B. Helicobacter infection in humans and animals. Comp Cont Educ Pract Vet. 2002. 24:688–696.
9. Fox JG. The non-H. pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut. 2002. 50:273–283.

10. Fox JG, Correa P, Taylor NS, Lee A, Otto G, Murphy JC, Rose R. Helicobacter mustelae-associated gastritis in ferrets. An animal model of Helicobacter pylori gastritis in humans. Gastroenterology. 1990. 99:352–361.

11. Fox JG, Lee A. The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals. Lab Anim Sci. 1997. 47:222–255.
12. Geyer C, Colbatzky F, Lechner J, Hermanns W. Occurrence of spiral-shaped bacteria in gastric biopsies of dogs and cats. Vet Rec. 1993. 133:18–19.

13. Hall JA. Ettinger SJ, Feldman EC, editors. Diseases of the stomach. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 2000. 5th ed. Philadelphia: Saunders;1154–1181.
14. Handt LK, Fox JG, Stalis IH, Rufo R, Lee G, Linn J, Li X, Kleanthous H. Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol. 1995. 33:2280–2289.

15. Happonen I, Saari S, Castren L, Tyni O, Hanninen ML, Westermarck E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats. Zentralbl Veterinarmed A. 1996. 43:305–315.

16. Henry GA, Long PH, Burns JL, Charbonneau DL. Gastric spirillosis in beagles. Am J Vet Res. 1987. 48:831–836.
17. Herbrink P, van Doorn LJ. Serological methods for diagnosis of Helicobacter pylori infection and monitoring of eradication therapy. Eur J Clin Microbiol Infect Dis. 2000. 19:164–173.

18. Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histopathological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995. 112:307–318.

19. Lage AP, Godfroid E, Fauconnier A, Burette A, Butzler JP, Bollen A, Glupczynski Y. Diagnosis of Helicobacter pylori infection by PCR: comparison with other invasive techniques and detection of cagA gene in gastric biopsy specimens. J Clin Microbiol. 1995. 33:2752–2756.

20. Leodolter A, Wolle K, Malfertheiner P. Current standards in the diagnosis of Helicobacter pylori infection. Dig Dis. 2001. 19:116–122.

21. Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 1968. 3rd ed. New York: McGraw-Hill;238–240.
22. Monteiro L, de Mascarel A, Sarrasqueta AM, Bergey B, Barberis C, Talby P, Roux D, Shouler L, Goldfain D, Lamouliatte H, Mégraud F. Diagnosis of Helicobacter pylori infection: noninvasive methods compared to invasive methods and evaluation of two new tests. Am J Gastroenterol. 2001. 96:353–358.

23. Pirarat N, Makbunsri T, Sukkamon S, Amornchailertrat S, Rungsipipat A, Sunyasootcharee B. The relationship between pathological gastric changes and Helicobacter spp. in dog. Thai J Vet Med. 2003. 33:73–80.
24. Ploskonosova II, Baranov VI, Gaziev AI. Estimation of DNA damage and repair in tissues of gamma-irradiated animals using the polymerase chain reaction. Biochemistry (Mosc). 1999. 64:1320–1325.
25. Queiroz DM, Rocha GA, Mendes EN, De Moura SB, De Oliveira AM, Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996. 111:19–27.

26. Riley LK, Franklin CL, Hook RR Jr, Besch-Williford C. Identification of murine helicobacters by PCR and restriction enzyme analyses. J Clin Microbiol. 1996. 34:942–946.

27. Simpson KW, Strauss-Ayali D, McDonough PL, Chang YF, Valentine BA. Gastric function in dogs with naturally acquired gastric Helicobacter spp. infection. J Vet Intern Med. 1999. 13:507–515.

28. Vaira D, Holton J, Menegatti M, Ricci C, Gatta L, Geminiani A, Miglioli M. Review article: invasive and non-invasive tests for Helicobacter pylori infection. Aliment Pharmacol Ther. 2000. 14:Suppl 3. 13–22.
29. Yamasaki K, Suematsu H, Takahashi T. Comparison of gastric lesions in dogs and cats with and without gastric spiral organisms. J Am Vet Med Assoc. 1998. 212:529–533.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download