Abstract
Telomere length maintenance is regarded as a fundamental step in tumorigenesis, as most human brain tumors, including meningiomas, stabilize the ends of their chromosomes using telomerase. This investigation represents an introduction to telomerase expression in canine and feline meningiomas. Twenty-five archived cases (14 dogs and 11 cats) were immunohistochemically tested for human-telomerase reverse transcriptase (h-TERT), scored, and quantified; furthermore, mitoses were counted on sections stained with a modified toluidine blue. The h-TERT antibody immunolabelled the nucleus and nucleolus of meningeal neoplastic cells, with an intensity ranging from mild to strong and a speckled distribution; a significantly higher expression in cats was noted, while no significant association between h-TERT immunolabelling and sex or histotype was evident in dogs or cats. The telomerase enzyme represents a fundamental parameter of potential malignant transformation, which may occur independently of the signal to proliferate, thereby supplying the cells with unlimited growth capabilities. Telomerase expression could be a prognostic indicator independent of the kinetic parameters, although this should be evaluated using a larger dataset with available clinical information.
Telomerase is a ribonucleoprotein complex that prevents the erosion of chromosomal extremities (telomeres) in eukaryotes. These sequences are composed of multiple Grich repeats and an abundance of associated proteins.
The telomerase enzyme is formed by an RNA component and a reverse transcriptase, the catalytic subunit which is considered to be the primary determinant for its activity [5]. The human-telomerase reverse transcriptase (h-TERT) immunohistochemistry (IHC) supplies an indirect indication of the presence of the enzyme.
Maintenance of telomere length is essential for tumorigenesis, as most human tumors stabilize their chromosome ends using telomerase [5]. Many types of tumors, including brain cancers, have been studied by utilizing these procedures. Meningiomas, which may arise anywhere along the meninges, are the most common types of primary brain tumors in domestic animals; they are usually discrete, slow growing masses with smooth surfaces and broad dural attachments [3].
Recently, a subset of 27 canine meningiomas was tested for telomerase immunoreactivity, and TERT expression was found to be significantly correlated with the MIB1 antibody labelling index [4].
The aim of this study was to expand the present knowledge regarding telomerase expression in both canine and feline meningiomas.
Twenty-five cases of meningiomas (14 canine and 11 feline) were selected and classified according to the World Health Organization (WHO) scheme [3].
Replicate sections were tested for the presence of the enzymatic catalytic subunit of telomerase, h-TERT. Endogenous peroxidase activity was blocked by immersion in 0.3% hydrogen peroxide in methanol for 30 min; slides were immersed in citrate buffer (pH 6.0), heated for two 5 min periods in a microwave oven at 750W. The primary antibody, an anti-human TERT (Clone 44F12, diluted 1 : 50 in PBS; Novocastra, UK), was applied to the slides overnight at 4℃, followed by a streptavidin-biotinperoxidase complex (LSAB Kit; Dako, Netherlands) and diaminobenzidine (0.04% for 7 min) as chromogen. Sections were then counterstained with Papanicolaou hematoxylin, rinsed in tap water, dehydrated, and mounted. An isotype-matched IgG negative control was run on each section. Canine normal testis was used as a positive tissue control.
The h-TERT index was assessed by an image analyzer (Lucia 32G/Mutech; Nikon, Japan) in ten fields (×20 lens) and expressed as the percentage of positive cells. Furthermore, h-TERT labelled cells were subjectively estimated using semiquantitative grading, in which 0 indicated the absence of positive cells, 1 was 1-10% of positive cells, 2 was 11-30% of positive cells, 3 was 31-60% of positive cells, and 4 was more than 61% of positive cells.
Mitoses were counted in five fields (×40 lens) on replicate sections stained with a toluidine blue technique, which was modified to enhance the detectibility of mitotic figures [1]. Mitotic activity was then graded as low (L): 0-5 mitoses, or high (H): >6 mitoses.
Parametric one-way analysis of variance (ANOVA) was performed to compare h-TERT values with histologic type, gender and species. Simple logistic regression analysis was used to evaluate the association between the two tested methods (subjective and automated).
A value of p < 0.05 was considered to be significant.
In canine meningiomas, the histotypes most often observed were meningothelial (7/14) and anaplastic (4/14), followed by fibroblastic (1/14), transitional (1/14), and psammomatous (1/14), with a strong predominance of females (9/14). Among cats, the transitional variety was mostly represented (6/11), followed by psammomatous (2/11) and anaplastic (2/11); a predominance of males was noted (7/11) (Table 1).
Concerning IHC, h-TERT protein was localized in the nucleus, notably the nucleolus, and was also occasionally found in the cytoplasm of neoplastic meningothelial cells (2 cases) (Fig. 1A-C) (Table 1). In three specimens out of 25, no signal was detected; in the remaining samples, the staining intensity ranged from mild to strong, being very variable from case to case. The distribution of immunoreactive cells was speckled throughout the slide; even the percentage was very variable, with the proportion of positive cells ranging from 0 to 90%. According to the adopted semiquantitative grading system of h-TERT immunopositivity, five cases were classified in group 0, seven in group 1, two in group 2, seven in group 3, and four in group 4 (Table 1).
The comparison of the two tested counting systems indicated a direct positive correlation between the two methods (r = 0.881; p = 0.0000).
h-TERT expression was higher in meningioma samples from cats than in those from dogs (automated count in dogs: mean 23.3%, range: 0.71-73.91%; automated count in cats: mean 42.3%, range 15.93-68.87%; p = 0.06). No other association was evident between h-TERT immunolabelling and sex or histotype for dogs or cats.
Modified toluidine blue staining (Fig. 2) was useful to appreciate a very low mitotic activity in the vast majority of meningiomas (L group: 21 out of 25); only four cases evidenced a high number of mitotic figures (H group: 4 out of 25), including three dogs (2 anaplastic, 1 meningothelial) and one cat (anaplastic meningioma) (Table 1).
No correlation between the number of h-TERT immunoreactive cells (telomerase expression) and their proliferative activity, assessed by mitotic count on toluidine blue-stained sections, was evident.
In our series, canine meningiomas were more frequently found to be of the pure meningothelial type than of other types, although the WHO classification schemes report the transitional variety to be the most commonly observed in dogs [3]; nevertheless, the transitional variant shows features of both meningothelial and fibroblastic meningiomas. Furthermore, meningiomas occurred more frequently in female dogs and male cats; this finding mirrors the results of other reports [11], and has been putatively related to a dissimilar expression of estrogen, progesterone, and androgen receptors in canine and feline meningiomas [7].
Telomerase expression, which is assessed by h-TERT IHC, was displayed in expected locations, mainly nucleolar and nuclear locations, as previously reported, and also was rarely found in cytoplasmic locations [9]. Strong nucleolar staining may be explained as the presence of the telomerase holoenzyme, which is assembled within the nucleolus. The nuclear labelling may represent the active telomerase complex at the ends of the chromosome. Conversely, the cytoplasmic staining may be interpreted as the shuttling of telomerase holoenzyme from the nucleus, out to the cytoplasm, and then back into the nucleus during the assembly process [4]. This machinery is well-known because the relevance of telomerase in the process of tumoral transformation has been investigated thoroughly. In fact, its activation is considered to be a fundamental step in the "immortalization" of neoplastic cells, and has been suggested to play a key role in the progression of several tumors including human intracranial meningiomas [6]. The data obtained in the present study also seem to support this trend in canine and feline meningiomas.
The correlation observed between the subjective semiquantitative grading and the automated count was highly significant, considering the two systems to be interchangeable in the evaluation of h-TERT IHC. With both measurement systems, there was no appreciable relationship between h-TERT expression and the meningioma histotype, or with other variables such as gender and age. The h-TERT expression tended to be higher in samples from feline meningiomas than in canine meningiomas, and this may suggest a different role of the telomerase pathway between the two species, and may indicate a different prognostic significance.
In most human and animal tumors, the proliferative activity of neoplastic cells is directly related to the biological behavior. For this reason, the proliferative activity is considered to be a relevant prognostic factor that can be assessed by the counting of mitoses, or by the immunohistochemical evaluation of proteins expressed throughout the cell cycle (i.e. Ki67 antigen recognized by MIB1 antibody) [10]. In this respect, arachnoid "cap" cells, from which meningiomas arise, have a slow rate of cell division [2], and therefore a high mitotic activity cannot be expected in their tumoral transformations. Noticeably, MIB1 immunostaining was ineffective in our series (data not shown). This behavior can be explained with prolonged storage of samples (in formalin or in paraffin blocks), which is responsible for subcellular modifications (i.e. cross-linking between proteins and/or nucleic acids that provides a strong steric hindrance creating an intricate physical barrier to antibodies employed in IHC) [8]. Therefore, the proliferative activity of tumor cells has been evaluated by counting mitoses in sections stained with a modified toluidine blue technique, which enhances the detectibility of mitotic figures. Most cases prompted a very low proliferative activity (L group: 21 out of 25); only four cases displayed relatively high mitoses counts, which suggested a more aggressive phenotype, and three of these were grouped in the anaplastic histotype, that, among other meningioma variants, behaves in an aggressive fashion and carries a much poorer prognosis than do benign meningiomas (malignant meningioma).
Several studies have attempted to assess the proliferative potential and the existence of an association between meningioma recurrence and MIB1 labelling index in patient groups with low and high risk of recurrence, attributing a prognostic significance to the mitotic index and to the Ki-67 labelling index [12]; however, the actual prognostic relevance of these parameters remains controversial, heterogeneous, and of uncertain value [10]. In this respect, it has been postulated that h-TERT expression might be a relevant marker of progression towards recurrence and malignancy of meningiomas [6].
In the present study, no significant association between h-TERT expression and the histological type of meningioma or the mitoses count was evident, although a recent paper reports an association between h-TERT and MIB1 staining in canine brain tumors (meningioma and oligodendroglioma) [4]. A possible explanation for this is that telomerase activation, a fundamental parameter of potential malignant transformation, may occur independently of the signal to proliferate, thereby supplying the cells with unlimited growth capabilities [6].
These preliminary findings suggest that telomerase expression could play a role as a prognostic indicator that acts independently of kinetic parameters, although this should be evaluated using a larger dataset with available clinical information.
Figures and Tables
Fig. 1
Dog. Immunohistochemical staining of h-TERT in the nucleus (A), nucleolus (B), and cytoplasm (C) of neoplastic meningothelial cells. DAB chromogen. Counterstain with Papanicolaou hematoxylin. A; × 40, B & C; × 63.
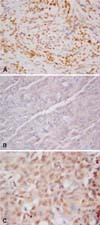
Table 1
Synopsis of signalment, histological classification, telomerase expression, and mitotic activity in meningiomas from dogs and cats*

*h-telomerase reverse transcriptase (h-TERT) staining pattern; distribution within nucleus (N), nucleolus (Nu) and cytoplasm (C). h-TERT labelling index; number of cells assessed by image analysis, and expressed as percentage of positive cells. h-TERT grading; subjective evaluation, 0 (no cells are labelled), 1 (1-10% of cells are labelled), 2 (11-30%), 3 (31-60%), 4 (more than 61%). Mitotic activity; number of mitoses counted in 5 fields (× 40), L (0-5 mitoses), H (more than 6 mitoses).
Acknowledgments
This work was partially supported by the Ministry of Education, University and Research of the Italian Government (Scientific Research Programs of Relevant National Interest, PRIN 2005).
References
1. Chieco P, Pagnoni M, Romagnoli E, Melchiorri C. A rapid and simple staining method, using toluidine blue, for analysing mitotic figures in tissue sections. Histochem J. 1993. 25:569–577.

2. Falavigna A, Santos JA, Chimelli L, Ferraz FA, Bonatelli Ad. Anaplastic meningioma: case report. Arq Neuropsiquiatr. 2001. 59:939–943.

3. Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers BA, van Winkle TJ. Histological Classification of Tumors of the Nervous System of Domestic animals. Second Series. 1999. Vol. 5. Washington DC: Armed Forces Institute of Pathology;27–29.
4. Long S, Argyle DJ, Nixon C, Nicholson I, Botteron C, Olby N, Platt S, Smith K, Rutteman GR, Grinwis GCM, Nasir L. Telomerase reverse transcriptase (TERT) expression and proliferation in canine brain tumors. Neuropathol Appl Neurobiol. 2006. 32:662–673.

5. Maes L, Kalala JPO, Cornelissen R, De Ridder L. Telomerase activity and h-TERT protein expression in meningiomas: an analysis in vivo versus in vitro. Anticancer Res. 2006. 26:2295–2300.
6. Maes L, Kalala JPO, Cornelissen R, De Ridder L. Progression of astrocytomas and meningiomas: an evaluation in vitro. Cell Prolif. 2007. 40:14–23.
7. Mandara MT, Ricci G, Rinaldi L, Sarli G, Vitellozzi G. Immunohistochemical identification and image analysis quantification of oestrogen and progesterone receptors in canine and feline meningioma. J Comp Pathol. 2002. 127:214–218.

9. Panarese S, Brunetti B, Sarli G. Evaluation of telomerase in canine mammary tissues by immunohistochemical analysis and a polymerase chain reaction-based enzyme-linked immunosorbent assay. J Vet Diagn Invest. 2006. 18:362–368.

10. Quiñones-Hinojosa A, Sanai N, Smith JS, McDermott MW. Techniques to assess the proliferative potential of brain tumors. J Neurooncol. 2005. 74:19–30.

11. Summers BA, Cummings JF, de Lahunta A. Summers BA, Cummings JF, de Lahunta A, editors. Tumors of the central nervous system. Veterinary Neuropathology. 1995. St. Louis: Mosby;351–401.
12. Torp SH, Lindboe CF, Granli US, Moen TM, Nordtomme T. Comparative investigation of proliferation markers and their prognostic relevance in human meningiomas. Clin Neuropathol. 2001. 20:190–195.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download