Introduction
Osteopontin (OPN) is a highly acidic glycoprotein that was initially isolated from the mineralized matrix of bovine diaphyseal bone [2,8]. Subsequently, it has been detected in several tissues, including the kidney [16], brain [10], cancers [13], and body fluids, including breast milk [7] and male seminal fluids [1]. Although the function of OPN depends on the type of cell in which it is expressed, its major roles are thought to be in cell adhesion [13] and cell activation/proliferation leading to the production of intracellular signals [14].
In the male reproductive system, OPN has been detected in a variety of male tissues including testis and epididymis as well as sperm [4,9,11,15], and it has been known to serve as one of decapacitation factors to prevent premature activation of sperm motility [3]. OPN messenger RNA has been specifically detected in developing germ cells that contained elongated spermatids in bull testis [9], in the Sertoli cells and germ cells of mouse testis [15], and in the Sertoli cells of rat testis [11]. The immunohistochemical findings largely match those of OPN mRNA expression in mouse [15] and rat [11] testis with some variation. By contrast, in the bull, OPN protein has been identified in the ampulla of the vas deferens and the seminal vesicles, but not in the testis or epididymis [1]. Furthermore, OPN has been identified in both germ cells and Sertoli cells in mouse testis [15] and in the early germ cells, but not in spermatids or Sertoli cells, of rat testis [5]. Therefore, there are distinct interspecies differences in the localization of OPN. Although cytology and duration of the cycle of the seminiferous epithelium of the boar was well documented [12], little is known on the expression of OPN in boar testes. The aim of this study is to examine the localization OPN in the boar testis to understand its involvement in spermatogenesis.
Materials and Methods
Testes from six-month-old mature Landrace pigs (n = 3) were obtained from a local slaughterhouse. The tissues were fixed in 10% buffered formalin for 48 h before being embedded in paraffin for histological examination. For comparison, the testes of one-day-old postnatal piglets (n = 3) were collected and processed for routine histology. Pieces of testes were frozen until used in Western blot protein analyses.
The frozen tissues were thawed at room temperature, minced, homogenized, and lysed in a buffer containing 40 mM Tris-HCl (pH 7.4), 120 mM NaCl, and 0.1% Nonidet P-40 (polyoxyethylene [9] p-t-octyl phenol) supplemented with the protease inhibitors leupeptin (0.5 µg/ml), phenylmethylsulfonyl fluoride (1 mM), and aprotinin (5 µg/ml). Equal amounts of protein (30 µg) were loaded in each lane and subjected to denaturing 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the proteins were electrotransferred onto nitrocellulose membranes (Schleicher & Schuell, USA), and blotted with rabbit anti-human osteopontin (code no. 100-401-404) (Rockland, USA). This antibody recognizes the full-length 66-kDa osteopontin protein, as well as the 32-kDa C-terminal fragments of thrombin and matrix metalloproteinase (MMP)-cleaved OPN (data sheet). The anti-OPN cross-reacts with OPN from the pig and dog. Subsequently, the bound antibodies were stripped from the membranes, which were reprobed with monoclonal antibody to beta-actin (Sigma, USA).
Briefly, 5-µm-thick sections of paraffin-embedded pig testes were deparaffinized before being exposed to citrate buffer (0.01 M, pH 6.0) in a microwave for 3 min. The sections were treated with 0.3% H2O2 in methyl alcohol for 20 min to block endogenous peroxidase activity. After three washes in phosphate-buffered saline (PBS), the sections were incubated with 10% normal horse or goat serum before being incubated with rabbit anti-OPN (1 : 1,000) for 1 h at room temperature. After 3 washes in PBS, the appropriate biotinylated secondary antibody and avidin-biotin peroxidase complex (Vector Elite; Vector, USA) were added sequentially. The peroxidase reaction was developed with diaminobenzidine as the substrate (Vector, USA). Before being mounted, the sections were counterstained with hematoxylin. For controls, each primary antiserum was omitted from some sections.
Results
Histology of boar testis
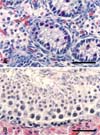
In postnatal piglets, hematoxylin-eosin stained sections showed undifferentiated seminiferous tubules and interstitial cells. Some gonocytes and undifferentiated Sertoli cells were visualized in the seminiferous tubules (Fig. 1A). In the adult boar testis, the seminiferous tubules contained primary spermatocytes, spermatids, and Sertoli cells at various stages (Fig. 1B).
Western blot analysis
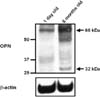
In postnatal piglets, the Western blot analysis detected the 66-kDa OPN band, but not the 32-kDa band (Fig. 2, left lane). Although additional bands were detected in the blots, these were thought to be the products of MMP-cleaved osteopontin. In the adult testis, the 66- and 32-kDa OPN bands were clearly identified (Fig. 2, right lane). The OPN immunoreactivity in adult testis was more intense than that in the postnatal testis, based on densitometric analysis (data not shown).
Immunohistochemical localization of OPN in the boar testis
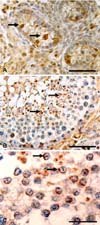
The cellular localization of OPN in the testis was evaluated immunohistochemically. In postnatal piglets, weak OPN immunoreactivity was observed in some undifferentiated cells in the seminiferous tubules, which consisted of 1 or 2 layers of cells. Intense OPN immunoreactivity was visualized in gonocytes, the precursors of spermatogonia, while it was weak in undifferentiated supporting cells (presumably Sertoli cells) in the seminiferous tubules (Fig. 3A). The OPN immunoreactivity in each cell type varied from tubule to tubule. OPN was also immunostained in interstitial Leydig cells.
In adult testis, the immunoreactivity of OPN varied with the seminiferous tubules. Some, but not all, seminiferous tubules showed OPN immunoreactivity.
In OPN+ seminiferous tubules, intense osteopontin immunostaining was seen in the residual bodies and acrosomes of spermatids (Fig. 3B & C), while weak OPN immunoreactivity was also seen in spermatogonia, preleptotene spermatocytes, and poachytene spermatocytes but in few Sertoli cells. In addition, some Leydig cells were immunopositive for OPN.
Discussion
The cellular localization of OPN protein in the boar testis matched that in mouse testis [15], but not in bull [1] or rat testis [5]. In brief, OPN immunostaining in the rat testis was localized to early germ cells from spermatogonia up to early pachytene spermatocytes, but not in Sertoli cells or spermatids [5]. An in situ hybridization study detected OPN signals in the basal and adluminal region of the seminiferous tubules in the rat testis, and it was postulated that OPN was a Sertoli cell product that played a role in spermatocyte adhesion [11]. Very recently, OPN protein was detected in the cytoplasm of Sertoli cells and luminal cells of the mesonephric tubules, with small amounts associated with the plasma membrane of germ cells in mice [15].
In this study, we found that OPN was intensely localized in the early and late germ cells, including spermatids, of adult boars. Considering the localization of OPN in the acrosomes and residual bodies in the adult porcine testis, we postulated that OPN plays a role in the early development of germ cells, as well as in the formation of acrosomes in spermatid development; ultimately, OPN aggregates in the residual bodies. This finding differs from that in bull testis [1]. We cannot exclude the possibility that some OPN in acrosomes is involved in the process of fertilization through adhesion or the decapitation of the ovum.
OPN has been detected in Sertoli cells in various animal species, including the bull [1], mouse [15], and rat [11], although a contradictory result was also reported in rats [5]. In this study, we found diffuse OPN immunostaining in undifferentiated supporting cells (presumably the progenitors of Sertoli cells) in the seminiferous tubules of newborn piglets. In the Sertoli cells of adults, OPN immunoreactivity was sparse, if any, with the immunostaining protocol used. Therefore, we postulate that OPN is expressed developmentally in undifferentiated supporting cells, and reduced in the Sertoli cells in adult boars. However, we do not exclude the possibility that OPN mRNA exists in Sertoli cells, as it occurs there in other animal species.
Functionally, there is a consensus that OPN plays a role in the adhesion of germ cells to the basement membrane and adjacent Sertoli cells because OPN is characteristically involved in cell adhesion. Therefore, we believe that OPN serves as an adhesive during germ cell development in both postnatal and adult testes, as shown in macrophages in inflammatory disease model [6].
Concerning OPN expression in Leydig cells, little is known of the functional role of OPN in endocrine cells. We postulate that OPN plays a role in cell signaling or cell adhesion between the interstitium of the testes.
Combining all the findings, we postulate that OPN appears early in the developing testis after birth, and contributes to both gonad development and spermatogenesis, possibly through the cell adhesion capacity of OPN and acrosomal formation, respectively. The functional role of OPN in the boar testis needs further study.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download