Introduction
Diabetes mellitus is one of the most common endocrine diseases in dogs. Type I or insulin-dependent diabetes mellitus (IDDM) is caused by the lack of insulin and results from the destruction of the insulin-producing beta cells in the pancreas [8]. The present treatment for IDDM includes careful monitoring of blood glucose levels, multiple injections of insulin, specialized diet and an exercise regimen.
Vanadium is a transitional element that is widely distributed in nature, with an atomic number of 23, atomic weight 50.9415 and oxidation states from 3+ to 5+. Its function in the body is not well understood. However, orally administrated sodium vanadate has been reported to improve DM in human diabetes before the discovery of insulin and its clinical use to treat DM has been previously demonstrated [3]. Vanadium compounds have been shown to be effective in animal models such as spontaneously diabetic (BB) rats, insulin-resistant Zucker fa/fa rats [4] and recently in human trials [3]. In addition, sodium vanadate has been proven a valuable adjunct to insulin in the treatment of diabetes mellitus [3,4].
The mechanism by which lowering of the blood glucose levels occurs is not understood. Evidence exists that the insulin-like actions of vanadate involve phosphorylation of the insulin receptor [12]. It has been suggested that the glucose-lowering effect of vanadium is due to its insulin-mimetic properties, such as increasing glucose transport [7], glycogen synthesis, glycolysis [6] and glucose oxidation in adipose tissue [6,10]. Moreover, vanadium has been reported to stimulate lipogenesis [11].
There are no prior reports on the glucose lowering effects of vanadium in diabetic dogs. Therefore, the purpose of this study was to assess the effects of treatment of vanadium salts in alloxan-induced diabetic dogs by comparing the blood glucose levels and serum chemistry profiles between a diabetic control group and a vanadium treatment group.
Materials and Methods
Preparation of experimental dogs
Clinically normal adult mongrel dogs without distinction of breed and sex were prepared for this experiment. Their body weight ranged from 2 to 6 kg initially. They were supplied dry food for adult dogs twice a day during this study, and given free access to sufficient tap water before the vanadium treatment study. They were adjusted to the new experimental environment adequately before the induction of diabetes mellitus.
Induction of diabetes mellitus
The dogs were fasted for 24 h prior to the induction of diabetes mellitus. Blood was collected for baseline data determination including: baseline glucose, fructosamine and serum chemistry profile (BUN, creatinine, ALT, AST, ALP, TG, cholesterol, electrolytes). Catheters were placed in the cephalic or jugular veins and a lactated Ringer's solution was infused at a maintenance dose.
Fresh solution of alloxan monohydrate (Sigma, USA) for diabetes mellitus induction was prepared just prior to injection [1,2]. Alloxan solution was made by dissolving it in normal saline at a concentration of 100 mg/ml, and filtered using a 0.22 µm syringe filter unit (MILLEX-GV, 0.22 µm syringe filter unit; Millipore SA, France). This prepared solution was given intravenously at a dose of 80~100 mg/kg of alloxan for the dogs immediately after preparation. Six hours after alloxan injection, the blood glucose levels were measured every 4 h until resolution of the hypoglycemia. If the blood glucose levels were too low, a glucose solution (5-10%) was given intravenously.
Selection of diabetic dogs
After injection of the alloxan solution, the blood glucose levels were measured frequently for days. Once the hypoglycemia resolved the fasting blood glucose levels were measured at 10 : 00 a.m. every morning. The dogs were evaluated for glycosuria once a day during the first stage. Diabetes was confirmed at one week after the alloxan injection by the presence of continuous fasting hyperglycemia (fasting blood glucose level >180 mg/dl) and persistent glycosuria. Only the dogs with induced diabetes mellitus and without acute renal failure or any other side effects were selected to continue the experiment.
Experimental design and oral administration of sodium metavanadate solution
The selected diabetic dogs were divided into two groups by random assignment. First, a diabetic control group (DC group, n = 4), was given normal adult dry pellets and tap water. The vanadium treatment group (DV group, n = 6) of diabetic dogs was treated with vanadium (96% sodium metavanadate; Acros Organics, USA). They were supplied the same dry pellets and housed in the same environment as the DC control group; however, the dogs in this group were given 0.1~0.2 mg/ml (w/v) of sodium metavanadate solution to drink for three weeks instead of tap water. All dogs had free access to the vanadium solution and water.
The sodium metavanadate solution was prepared by dissolving it in purified water. Alloxan-treated dogs were given aqueous solutions of sodium metavanadate beginning one week after the confirmation of diabetes mellitus, and this continued to be supplied for three weeks.
Blood samples were collected from the jugular vein every week before and after alloxan injection and vanadium administration for four weeks. The BUN, creatinine, ALT, AST, ALP, triglyceride, cholesterol and electrolytes were measured. They were analyzed using an automatic serum chemical analyzer (VetTest 8008; IDEXX Lab, USA) and automatic electrolytic analyzer (VetLyte; IDEXX Lab, USA). Fructosamine levels were also determined.
Pathological findings
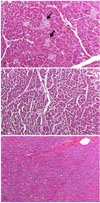
After death or euthanasia, one dog in each group was selected for necropsy. In addition, one normal dog was sacrificed for necropsy to compare the DC and DV groups. The internal organs such as pancreas, kidney, liver were fixed in formalin solution and stained with H&E and then examined for the microscopic morphology.
Results
The fasting blood glucose levels and fructosamine levels were measured every week. The former are presented in Fig. 1, and the latter are shown in Fig. 2. As the data shows, the blood glucose levels of both groups were within normal range before the injection of alloxan, however it increased after one week from the onset of the injections, and remained high. With the maintained hyperglycemia, the dogs showed polyuria, polydipsia, polyphagia, glycosuria and weight loss; although the clinical signs related to the diabetes mellitus were not obvious. Therefore, the induced diabetes mellitus was confirmed by the continuous hyperglycemia, glycosuria and clinical signs.
The blood glucose levels in the DV group were significantly lower compared to the DC group at 2, 3 and 4 weeks after the injection of the alloxan (p < 0.01). The fructosamine levels showed a similar pattern to the blood glucose levels in both groups. The fructosamine levels of the DV group were also significantly lower than those of the DC group at 2, 3 and 4 weeks (p < 0.05). In addition, for the dogs in the DV group, the food and water intake were decreased and the volume of urine decreased compared to the dogs in the DC group, although the exact data were not obtained.
Selected chemical parameters during the one month study following alloxan administration are summarized in Table 1. The serum chemistry profiles were not significantly different in comparisons between the two groups, except for the cholesterol levels. The cholesterol levels in the DV group were significantly lower than those in the DC group (p < 0.05). This may have been caused by the low blood glucose levels in the DV group causing normalization of lipid metabolism.
Necropsy was performed after euthanasia, or death in one dog, in the individual group and in one normal dog. The pancreas was examined mainly, especially the pancreatic islets. The histopathology examination showed that pancreatic islets disappeared and that the exocrine tissue remained relatively unaffected; any remaining islets were considerably atrophied compared to the normal pancreas (Fig. 3). These findings indirectly show that the blood glucose levels in the DV group may have been lowered not by insulin but by vanadium; this is because almost all of the pancreatic islet beta cells were destroyed and therefore resulted in insulin deficiency. The kidneys and livers were also examined. Necrosis of epithelial cells was observed in the tubules around the glomerulus of the kidney and glycogen degeneration was observed in the hepatic cells of both groups (data not shown). The damage to the kidneys may have been due to the alloxan, and glycogen accumulation in the hepatic cells may have been caused by the diabetic condition.
Discussion
The results of this investigation demonstrated that administration of a single dose of alloxan to dogs produced a reproducible model of diabetes mellitus that had minimal beta cell activity, and elevated glucose and cholesterol levels. However, the high mortality among the experimental dogs and the evidence of toxicity to the kidney and liver in addition to the pancreas was a serious problem. Many of the dogs developed symptoms such as emesis, hypersalivation, diarrhea or soft stool even without azotemia. The hypoglycemia 6 h after injection caused extremely adverse effects, perhaps related to severe beta-cell destruction. The extent of DM induction was variable. This might indicate that susceptibility of dogs to the beta-cytotoxic effects of systemic alloxan administration varies. In this experiment, the lactated ringer's solution was infused for a prehydration maintenance dose before the injection of the alloxan solution. It likely led to fluid diuresis by enhancing renal perfusion and resulted in a reduced renal toxicity from the alloxan.
The mechanism explaining the hypoglycemic effects of vanadium is not well understood; however, several hypotheses have been proposed. Because vanadium behaves like phosphate, the biochemistry of vanadium has been shown to inhibit protein phosphotyrosine phosphatase, which in turn stimulates protein tyrosine phosphorylation [12]. Thus, vanadium activated autophosphorylation of solublized insulin receptors similar to insulin action. Vanadium also stimulated the tyrosine kinase activity of the insulin receptor beta subunit. In addition, both vanadate and vanadyl have been found to be effective in stimulating glucose metabolism in rat adipocytes [4]. The concentration of vanadium solution was chosen based on a previous report that demonstrated a pronounced glucose-lowing effect in STZ-diabetic rats [11]. During administration, side effects were observed in many of the vanadium treated diabetic dogs. These dogs showed symptoms of lethargy, weight loss, mucous and/or hemorrhagic diarrhea, and refusal to eat and drink, especially during the first stage when vanadium water was provided for drinking. Seven dogs died or were sacrificed after drinking the vanadium water without renal dysfunction (normal BUN/Creatinine, normal production of urine); we thought that vanadium toxicity may have played a role, although this was not confirmed. These dogs were excluded from the study. All other vanadium treated dogs were healthy and tolerated the vanadium drinking water; their GI signs resolved progressively, without overt indication of toxicity during the three week treatment period. Moreover, the vanadium treatment did not significantly elevate the BUN, creatinine, ALT, AST, ALP levels in the DV group.
The most serious problem of the vanadium was its side effects. However, new compounds of vanadium are under development. These new compounds will be able to potentiate insulin-like effects with decreased undesirable side effects. Once these problems are resolved further use of this compound may become more clinically relevant for research and treatment of diabetes mellitus.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download