Introduction
Kidney transplantation is the ideal treatment for chronic renal failure and various end-stage renal diseases [4,13,20]. The viability of the transplanted organ depends its ability to tolerate cold and warm ischemia and reperfusion during surgery [6,10]. Ischemia-reperfusion injury is a major cause of renal failure and renal graft rejection. Therefore, reducing the extent of this injury in renal transplant patients is important for achieving a good prognosis.
Renal ischemia-reperfusion (I/R) injury leads to the production of excess reactive oxygen species (ROS) and reactive nitrogen species (RNS). These species cause oxidative stress resulting in alterations in the level of mitochondrial oxidative phosphorylation, ATP depletion, increases in the intracellular calcium and activation of protein kinases, phosphatases, proteases, lipases and nucleases leading to a loss of cellular function and integrity [21]. Therefore, it is important to reduce the levels of these hazardous metabolites in order to improve the patient's outcome.
In order to reduce these metabolites, many studies have examined a variety of free radical oxygen scavengers. These include the effects of external supplementation of antioxidants [3,8,12,13] and the activities of endogenous enzymatic antioxidant defense system in a kidney ischemia/reperfusion injury [2,7]. Ascorbic acid has been used to protect against corneal damage by free radicals in rabbits [19]. In addition, it has also been used to improve the renal hemodynamics as well as decreased oxidative stress, inflammation and fibrosis in the ischemic kidney of pigs [5]. Ascorbic acid is an inexpensive low-priced antioxidant that can be administered easily as it is water-soluble. This study investigated of the ability of ascorbic acid as free radical oxygen scavenger to attenuate ischemia-reperfusion injury and the recovery of the renal function in a canine renal autograft model.
Materials and Methods
Animals and experimental groups
Adult beagle dogs of both genders, weighing 10-13 kg (Marshal Farms, USA) were used in this study. The animals were acclimatized and maintained on a standard diet, a routine lighting cycle and room temperature for 6 months, and had a normal renal function before the surgical procedure. The dogs were assigned randomly into a control group (n = 4) and ascorbic acid treatment group (n = 4).
Kidney harvest
The left kidney was freed from the perirenal tissue and fat, and the left renal artery and vein were then clamped using an atraumatic vascular clamp. The ligation and resection of the vessels was performed at the aorta and caudal vena cava, and moved to a tray filled with ice.
Flushing
After the left kidney had been removed, all the arteries in the control group were perfused with cold normal saline. Cold normal saline and ascorbic acid (100 mg/kg; Dai Han Pharm, Korea) were perfused in the treatment group followed by autotransplantation.
Transplantation
After an overnight fast, the animals are premedicated with atropine sulfate (0.04 mg/kg, SC; Huons, Korea), and an antibiotic prophylaxis with cefazolin sodium (20 mg/kg, IV; Chong Kun Dang, Korea) and an analgesic with meloxicam (0.2 mg/kg, IV; Boehringer Ingelheim, Korea) were administered at induction. The animals were induced with thiopental sodium (12.5 mg/kg, IV; Dai Han Pharm, Korea) and maintained with isoflurane 2% and a 100% oxygen supply during the procedure. All the dogs were administered a balanced electrolyte solution (10 ml/kg/h, IV). The dogs were administered mannitol (1 g/kg, IV; Dai Han Pharm, Korea) 20 min before the nephrectomies. The kidneys were exposed through a midline incision, and the left renal artery, vein, and ureter were mobilized for transplantation. The kidneys were harvested, flushed with a cold, heparinized saline solution, and placed in a cold, sterile saline solution before the anastomosis. The renal vein was anastomosis to the caudal vena cava in an end-to-side manner using a simple continuous suture pattern of 6-0 polypropylene. The renal artery was anastomosed to the external iliac artery in an end-to-end manner using 7-0 polypropylene in a simple interrupted pattern. If 2 renal arteries were present, both arteries were managed with a bridging of the renal arteries using a modification of the technique reported by Sarin et al. [20]. The ureter was sutured to the bladder mucosa in a modified ureteroneocystostomy technique using 5-0 polyglyconate in a simple interrupted pattern. A nephropexy was created by suturing the renal capsule to the abdominal wall with simple interrupted sutures made from 4-0 polyglycolic acid. After implanting the autograft, contralateral kidneys were removed. Postoperatively, all the dogs were allowed access to water and food ad libitum.
Three days after surgery, vitamin C (30 mg/kg) was injected intravenously in treatment group with the same amount of vehicle (physiological saline solution) being injected in the control group.
Renal function
The blood urea nitrogen (BUN) and creatinine levels were determined from the serum samples taken on days 0, 1, 3, 5, and 7 before and after the procedure from the jugular vein, using a commercially available kit (VetTest; IDEXX, Japan). The results are expressed in milligrams per deciliter.
Blood Pressure
The invasive blood pressure was measured using Pulscan-Component (Scionic, USA) and a monitoring-set was used for the arterial blood pressure measurement (B/Braun, Germany) at the renal and external iliac arteries. The blood pressure was measured at pre-anastomosis, 10 and 60 min after the anastomosis through a direct invasion using a 24 G scalp vein set (Korea Vaccine, Korea) in the lumen of the artery.
Antioxidant enzyme activity in plasma
Blood samples were collected using an anticoagulant as EDTA, and centrifuged at 700~1,000 × g for 10 min at 4℃. The samples were then pipetted off the top yellow plasma layer without disturbing the buff layer, and the plasma samples were stored on ice until assayed or were freeze at -80℃. The superoxide dismutase (SOD) activity was determined using a commercial SOD assay kit (Cayman, USA) for measuring the SOD activity from the plasma. The activity was recorded spectrophotometrically at 450 nm. The enzyme activity was calculated as U/ml. The glutathione peroxidase (GSHPx) activity was measured using a commercial GSHPx assay kit (Cayman, USA). The activity was recorded spectrophotometrically at 340 nm. The catalase (CAT) activity was measured spectrophotometrically at 540 nm using a commercial CAT assay kit (Cayman, USA).
Histopathological examination
The study protocol called for euthanizing the surviving canines after the third post-transplant week if the serum creatinine values had normalized to <1.8 mg/dl. The tissue samples from the left kidney were taken for a histology examination after euthanasia on postoperative day 21. The tissue samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned in 4 µm slices, and mounted on slides. After deparaffinizing, each specimen was stained with hematoxylin and eosin for an optical microscopy examination. The morphological characteristics of the kidneys were determined using a blinded histology examination.
Statistical analysis
All the values are expressed as a means ± SD of the determinations for all the dogs in the group. The data was analyzed using an analysis of the variance followed by 2-way repeated measures analysis (ANOVA) and then by a Student's t-test. A p value <0.05 or 0.01 was considered significant.
Results
Renal function
The serum creatinine levels, which are an index of the kidney function, increased significantly to 6.73 ± 2.12 mg/dl in the control group (baseline levels; 0.72 ± 0.07) after 5 days of reperfusion and then decreased gradually. On the other hand, the serum creatinine levels in the treatment group (baseline levels; 0.87 ± 0.06) increased to 2.71 ± 1.32 mg/dl after 3 days reperfusion and then decreased gradually by 7 days. The BUN was also measured as a second index of the kidney function. Similar to the serum creatinine level, the BUN levels in the control group (baseline levels; 11.7 ± 6.68) increased significantly to 176.6 ± 49.32 mg/dl by 5 days after reperfusion. The BUN levels in the treatment group (baseline levels; 14.4 ± 1.26) increased to 59.87 ± 21.04 mg/dl after 7 days reperfusion.
Blood Pressure
The blood pressure was measured directly at the external iliac artery. The systolic blood pressure in the control group increased from 86.5 ± 19.67 to 98.5 ± 11.32 mmHg while in the treatment group, these levels decreased from 86 ± 12.67 to 84.75 ± 15.32 mmHg. In the control group, the diastolic blood pressure also increased from 77.75 ± 18.24 to 88 ± 11.51 mmHg while these levels decreased from 78.5 ± 12.23 to 77 ± 13.83 mmHg in the treatment group. In the control group, the mean blood pressure increased from 80.5 ± 18.92 to 91.75 ± 11.14 mmHg by 60 min after the anastomosis. In contrast, in the treatment group, the mean blood pressure increased to 85.5 ± 15.69 mmHg by 10min and then decreased gradually thereafter, and was almost normalized to 79.5 ± 14.20 mmHg by 60 min. However, there was no significant difference between the groups.
Antioxidant enzyme activity in plasma
The antioxidant enzyme activity in the plasma after the renal autotransplantation was evaluated. The specific SOD, GSHPx and CAT activities before surgery and 72 h after reperfusion are expressed as the mean ± SD. The activity of SOD increased slightly from 2.14 ± 0.22 to 2.31 ± 0.17 nmol/min/ml in the control group. In contrast, the activity of SOD in the treatment group increased from 2.37 ± 0.21 to 2.69 ± 0.25 nmol/min/ml by 72 h of reperfusion (p = 0.037, Fig. 1). There was no significant change in the GSHPx activity in the control group. On the other hand, in the treatment group, the level of activity increased significantly from 109.16 ± 19.51 to 132.47 ± 15.50 nmol/min/ml by 72 h of reperfusion (p = 0.032, Fig. 2). There was no significant change in the CAT activity in the control group whereas the CAT activity in the treatment group increased from 2.73 ± 0.30 to 3.74 ± 0.37 nmol/min/ml by 72 h of reperfusion (p = 0.038, Fig. 3).
Histology and morphologic examination
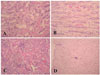
At the autopsy 21 days after the transplant, the control kidneys in which a normal saline had been administered demonstrated moderate cystic dilation of the tubules along with inflammatory cell infiltration, regeneration of the tubules and congestion (Fig. 4A & B). In contrast, the kidneys in the treatment group, which had been administered ascorbic acid after the renal transplantation showed slight damage to the tubules. The tubular epithelium was essentially normal, even though there was occasional, mild cystic dilation of tubules with mild peritubular inflammatory cell infiltration and regeneration of the tubules (Fig. 4C & D).
Discussion
Oxidative stress is an imbalance between oxidants such as ROS and antioxidants [22], and probably contributes to the development, progression, and complications of both acute renal and chronic renal failure, which is characterized by the increased production or decreased elimination of antioxidants [1,9,15,23,25]. Ascorbic acid reduces the level of reactive oxidant species both intracellularly and extracellularly, and maintains transition metals in their reduced form. In addition, ascorbic acid may quench the free radical intermediates of the carcinogen metabolism. Ascorbic acid is an outstandingly powerful antioxidant that reacts rapidly with a variety of oxidants, including the rather poorly reactive superoxide anion radical [11].
These results suggest that the levels of various antioxidant enzymes (SOD, GSHPx, and CAT) levels, which protect against oxygen free radicals, were higher in the ascorbic acid treated group. Generally, the conversion of the superoxide anion and hydrogen peroxide was impaired due to the decreased levels of SOD, GSHPx and CAT, resulting in an increase in the level of oxygen free radicals [16]. Therefore, the elevated superoxide and hydrogen peroxide levels accelerate the damage to the kidney. However, ascorbic acid as an antioxidant detoxified the hydrogen peroxide produced. In this study, ascorbic acid as an exogenous antioxidant appeared to attenuate I/R injury by increasing the activities of SOD, GSHPx, and CAT. On the other hand, the concentration of antioxidant enzymes increased due to a decrease in their consumption rather than decreased as a result of another scavenger.
In this study, although the invasive blood pressure was similar in both groups, the levels in the treatment group had almost normalized by 60 min after the transplant. There is evidence suggesting that vitamin C may affect the intracellular levels of glutathione, which can improve flow-mediated, endothelium-dependent dilation [29]. Ascorbic acid is a potent aqueous phase antioxidant that has been shown to improve the endothelial dysfunction as a result of an interaction between endothelium-derived NO and ROS [14,26,27]. Although the NO concentration was not measured in this study, it is believed that ascorbic acid can increased the quantity of NO under these conditions.
Bilateral multiple renal arteries were encountered during surgery. According to the literature, approximately 15% of humans have bilateral multiple renal arteries [18]. In this study, 37% of the dogs had double arteries, and all were different sized parallel vessels. Therefore, the technique of bridging the renal arteries was used in this study, and at autopsy, no complications from the arterial stenosis were observed. However, the possibility that multiple renal arteries could have affected the clinical outcomes cannot be excluded. Therefore, a study using more cases as well as a long-term follow-up will be needed.
The histology and morphological examination showed less damage to the tubules in the treatment group than in the control group. It has been reported that an inflammatory response induced by ischemia followed by reperfusion is largely responsible for the tissue damage observed [24,28]. In this study, an inflammatory response demonstrated slight damage to the tubules in the treatment group. Although irreversible damage of tubular system was observed in some dogs in both groups, it is unclear if it was due to surgical problems or to the effects of ascorbic acid. Therefore, further study with more samples will be needed. Although these results cannot explain the entire mechanism for the attenuation of ischemia-reperfusion injury, the results of the functional parameters, histopathological changes, antioxidant enzyme activity suggest that ascorbic acid alone may play a role in attenuating ischemia-reperfusion injury and assist in the recovery of the renal function after a renal transplant.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download