Abstract
Paratuberculosis (PTB) is a major disease problem worldwide, and causes major economic losses in the dairy industry. Although PTB has been reported in Korea, no studies have been conducted to determine its prevalence and no program has been developed to control the disease. In this study, the sera of beef (n = 1,056) and dairy cattle (n = 1,105) from all provinces in Korea were tested to determine the prevalence of PTB using two different ELISA: an 'in house' modified absorbed ELISA (P-ELISA) based on sonicated antigen from Mycobacterium avium subsp. paratuberculosis ATCC 19698, and a commercial ELISA (C-ELISA). Receiver operating characteristic analysis was used to determine the cutoff point for P-ELISA. Based on C-ELISA results, the area under the curve for P-ELISA was 0.913 (95% CI, 0.883 to 0.943). Using a cutoff point of 0.100, P-ELISA showed a sensitivity of 62.0% and a specificity of 93.7%. The kappa value and the percent agreement between the two ELISAs were 0.322 and 92.5%, respectively. Both ELISAs showed a significant correlation between age and seropositivity (p < 0.01). According to C-ELISA, 71 of 2,161 sera (3.3%, 95 CI, 2.6% to 4.1%) were test-positive. The national true prevalence of PTB was estimated to be 7.1%. The findings suggest that a control program should be implemented to limit the spread of this disease, and that P-ELISA could be used as a screening test that produces results similar to C-ELISA.
Paratuberculosis (PTB), Johne's disease, is a chronic progressive disease of ruminants caused by infection with Mycobacterium avium subsp. paratuberculosis (MAP). Although infection usually occurs in the first few months of life [35], the first sign of clinical disease may not appear until 6 months to 15 years post infection [5,23]. This long latent period has been attributed to control difficulties because subclinically infected cows become transmitters of PTB, and shed causative bacteria in feces before progressing to the terminal disease stage.
PTB causes substantial economic loss to the beef and dairy industries [4,19,29]. Therefore, an appropriate control program should be implemented to reduce the negative impact of PTB. Moreover, the initial step required for the successful development of a control program is the determination of the regional distribution of infected herds. Although cultivation of MAP from fecal or tissue samples is considered the reference test for PTB, it is a cumbersome and expensive method for detecting infected animals, especially when the numbers involved are large. Moreover, culture requires up to six months, and the method is not sufficiently sensitive to detect animals early in the course of infection. ELISA provides an alternative; it is faster (results take two to three days), provides increased sensitivity, and importantly is less expensive and can be used to test large numbers of animals [6]. For this reason, the authors developed an 'in house' absorbed ELISA method (P-ELISA) as a screening test, and compared this with a commercial ELISA (C-ELISA) using field samples from all provinces in Korea, excepting Jeju-do. P-ELISA yielded results similar to those obtained using C-ELISA. This study provides first data on the prevalence of MAP in Korea, information that will prove invaluable for the development of a national strategy to control the disease.
Sera were randomly collected by the National Veterinary Research and Quarantine Service as part of an annual investigation of bovine infectious diseases. A total of 2,161 bovine sera samples from 1,056 beef cattle in 448 farms and 1,105 dairy cattle in 219 farms, were collected from eight provinces (Gyeonggi, Gangwon, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk and Gyeongnam) in Korea from September to November in 2002.
All sera were tested using a commercial ELISA kit (Parachek; CSL, Australia) according to the manufacturer's instructions. Briefly, samples were diluted 1 : 20 in green diluent containing M. phlei, and then transferred to 96 well plates in duplicate. Positive and negative control solutions supplied by manufacturer were also tested in duplicate in each plate to validate test results. After washing, secondary antibody was diluted 1:100 in blue diluent. TMB was used as substrate. Optical density (O.D.) values were measured using an ELISA reader (Tecan, Australia) at 450 nm. The cutoff value for positive sera was defined as the mean of negative controls plus 0.100.
MAP ATCC 19698 was grown in Watson-Reid medium [36] at 37℃ for 12 wks. Bacterial cells were washed twice in phosphate buffered saline (PBS, pH 7.4) and resuspended in PBS. Cells were then sonicated twice on ice for 30 min, and centrifuged at 20,000 × g (Beckman, UK) at 4℃ for 30 min. Supernatant was then harvested and filtered using a 0.2 µm pore size filter. This filtrate was used as a capture antigen after measuring its protein concentration by spectrophotometry (Eppendorf, Germany). M. phlei was cultured in Dorset-Henley medium at 37℃ for 8-10 wks, and then prepared as described above for use as an absorption antigen.
Polystyrene ELISA plates (Maxisorp; Nalgen Nunc International, USA) were coated with 0.4 µg of capture antigen in 100 µl of 50 mM carbonate buffer (pH 9.6), and incubated overnight at 4℃. Coated plates were washed once with 100 µl of PBS (pH 7.4) containing 0.05% Tween20 (PBST), and incubated with 300 µl of 1% bovine serum albumin (BSA) in PBST for 2 h to block non-specific binding. Test sera were absorbed at a dilution of 1 : 20 in absorbent diluent (150 µg/ml of M. phlei, 5% fetal bovine serum, 2% BSA in PBST) and incubated for 30 min. After blocking, the plates were washed with PBST and incubated with absorbed sera (100 µl/well, in duplicate) for 30 min. Positive and negative controls were included in each plate. After washing, 100 µl of a 1 : 1500 dilution of horse radish peroxidase-labeled goat anti-bovine IgG (H + L) (Kirkegaard & Perry Laboratories, USA) was added to each well. Plates were then incubated for 30 min, washed 3 times, and 100 µl of peroxidase substrate (ABTS; Kirkegaard & Perry Laboratories, USA) was added. This reaction was stopped using 50 µl of 1M HCl, and plates were read in an ELISA reader (Tecan, Australia) at 405 nm.
Serum from a seropositive and fecal culture positive cow was used as a positive control, a serum pool from four seronegative animals from different herds, which had been seronegative and culture negative for more than 2 years, was used as a negative control. All steps were conducted at room temperature except the antigen coating step.
The receiver operating characteristic (ROC) analysis [3], kappa statistics [2], and percent agreement were used to compare P-ELISA with C-ELISA. Percent agreement (P) was defined as according to Eq. 1 [33],
P = (a + d) / n × 100,
where 'a' is the number of positive reactions, 'd' is the number of negative reactions, and 'n' is the number of total samples tested.
The test prevalence of PTB was calculated by dividing the number of positive sera by the number sera tested. This value was then adjusted to calculate the estimated test-positive prevalence (etp) at a nationwide level. The calculation takes into account bias due to different sample sizes and populations in the different provinces, as detailed by Eq. 2 [17],
where 'B1' is the number of total beef cows, 'D1' is the number of total dairy cows, 'p1' is the proportion of positive beef cattle, and 'q1' is the proportion of positive dairy cows in province 1. To calculate the estimated true prevalence (ETP) in Korea, etp was adjusted to compensate for the lack of sensitivity (Se) and specificity (Sp) of C-ELISA using Eq. 3 [24],
ETP = (etp + Sp - 1) / (Se + Sp - 1)
National population data were obtained from the Ministry of Agriculture and Forestry in Korea (28). All statistical analyses were carried out using commercially available software (Analyse-it, UK)
Based on C-ELISA results, ROC analysis was performed to analyze the efficacy of P-ELISA as a screening test and to determine a suitable cutoff value. Area under the curve (AUC) and the standard error of AUC were 0.913 [95% confidence interval (CI), 0.883 to 0.943] and 0.015, respectively (Fig. 1). Generally, the determination of an optimal cutoff value for the differentiation of a positive and negative reaction is difficult since the O.D. values of samples are not clearly divided into two groups. For this reason, after measuring Se and Sp of P-ELISA at various cutoff values (Table 1), a cutoff point of 0.100 was arbitrary chosen for further analysis, because this point gave relatively high Se and Sp values for P-ELISA, and this cutoff was then used to differentiate positive and negative sera in preliminary experiments (data not shown). This cutoff value was two times higher than that of the negative controls, and higher than the mean of negative controls plus standard variation (data not shown). Based on P-ELISA results using a 0.100 cutoff point, the kappa value and percent agreement between P-ELISA and C-ELISA were 0.322 and 92.5%, respectively (Table 2).
We also obtained information on the ages of 1,650 of the 2,161 cattle tested. Although P-ELISA detected about twice as many seropositive cows than C-ELISA, both ELISAs showed that a significant correlation existed between age and a positive response (Fig. 2).
C-ELISA test results revealed that 71 of 2,161 cows (3.3%, 95% CI, 2.6% to 4.1%) were seropositive (Table 3). Thus, based on the recently reported Se (28.4%) and Sp (99.7%) of C-ELISA [9] and the known population size, the national ETP was estimated to be 7.1% (Eqs. 2 and 3). Moreover, the proportion of seropositive dairy cattle was significantly greater than that of beef cattle (p < 0.01), i.e., 7 of 1,056 beef cattle (0.7%, 95% CI, 0.3% to 1.4%) versus 64 of 1,105 dairy cattle (5.8%, 95% CI, 4.5% to 7.3%). In terms of comparisons between provinces, Gyeonggi showed the highest proportion of seropositivity in total and beef cattle. Gangwon had a higher proportion of seropositive dairy cattle than other provinces. For beef cattle, all provinces, except Gyeonggi (6.3%), showed less than 1% seropositivity (Table 3).
To evaluate the efficacy of P-ELISA, it should be compared with other reference methods, such as bacterial cultures or other antibody based tests. However, since it was not possible to obtain fecal or tissue samples from the cows tested, C-ELISA, which has been evaluated for detecting infected cows in several studies [8,10,27], was used as the reference method in this study. For ELISA tests, the general problem encountered is the degree of signal overlap of samples from diseased and non-diseased animals, especially for PTB. Moreover, the Se and Sp values of ELISAs have been found to vary depending on cutoff point. Therefore, the cutoff of 0.100 for P-ELISA was chosen for further analysis for the reasons mentioned above in Results.
Using a cutoff of 0.100 for P-ELISA, the kappa value for the two ELISAs showed a low level of agreement, which has been reported for ELISAs previously in terms of detecting antibody to MAP [9,12,34]. This low agreement may have been caused by the presence or absence of certain antigenic components that react with some specific or crossreactive antibodies. Moreover, specific test antigens are the most important component of sensitive and specific ELISA tests. However, MAP is known to have antigenic components in common with other species of mycobacteria, and with related organism such as Corynebacterium spp., Norcardia spp., Actinomyces spp., and Eschericia coli [5,16,37]. In addition, different Sps of absorbed ELISAs for PTB were found in serum samples from different regions, which may reflect regional cross-reactive antibodies [31]. Our findings indicate that some cross-reactive antibodies remained after absorbing sera with M. phlei, and that this affected the Sps of the two ELISAs. In addition, although the capture antigens were prepared from the same organism, the antigenic composition of MAP preparations can be different depending on the method of preparation [10,15]. For these reasons, each of the ELISAs used in the present study might have only detected a subset of specific or cross-reactive antibodies. Nevertheless, the high AUC and percent agreement [33], and the similar age distribution patterns observed demonstrated that P-ELISA can be used as a herd screening test and as a pre-screening test for individual and followed with other identification tests, such as PCR or bacterial culture.
We tested cows up to six years of age, and both ELISAs revealed a significant correlation between animal age and a positive result. PTB is characterized by its long latent period, and thus, seroconversion is more readily detected in older animals. Thus, although cows are likely to be infected with MAP whilst young, most infections go undetected. Other studies have yielded similar results, although these studies also found that seropositivity is reduced in animals over six years of age [13,14], which may be due to the culling of symptomatic cows.
To date, few studies have been performed on PTB in Korea, and these have been limited in scope [20-22]. The present study is the first seroprevalence study conducted on PTB at a nationwide level. Only C-ELISA results were used to estimate of prevalence because this test has been used worldwide and well evaluated. In the present study, although the overall seroprevalence of PTB in Korea was found to be low to moderate compared with those of other countries [1,11,14,18,26,30,32], some provinces showed much higher seroprevalences. Many factors probably contribute to differences in prevalences between provinces, such as herd characteristics, climate, and environment effects. For example, Gangwon has been known by veterinarians to be an endemic region for PTB, and number of overpopulated herds in Gyeonggi may have contributed to this high seropositivity. In terms of dairy and beef cattle, our data reveal that the seropositive rate of beef cattle is significantly lower than that of dairy cattle (p < 0.01), as was previously reported by Kim et al. [21], which suggested a low overall prevalence in beef cattle in Korea. Similar results have also been reported in other countries [11,25]. This finding may be due to restricted transmission opportunities among beef cattle because they are culled earlier than dairy cattle.
Taken together, our data provide a general indication of the true state of PTB in Korea, and suggest that a national control program should be considered to control the disease. Our findings also suggest that different control systems might be needed in different provinces depending on the prevalence of PTB with consideration of the economic models of Johne's disease [7], and that programs should focus on limiting the spread of PTB among provinces. As a first step in any control program, large numbers of cattle should be tested, and due to its low cost and accuracy, we suggest that P-ELISA can be used for this purpose as a screening test for infected herd or for individual animals followed by other methods to verify PTB infection.
Figures and Tables
Fig. 2
Proportion of seropositive cattle determined by C-ELISA and P-ELISA by age. All cows under two years of age are included in the two year old group.

Acknowledgments
This study was funded in part by the Brain Korea 21 Program for Veterinary Science and the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University. Further support was provided by the Korean Research Foundation (Grant No. KRF-2006-005-J02903).
References
1. Adaska JM, Anderson RJ. Seroprevalence of Johne's-disease infection in dairy cattle in California, USA. Prev Vet Med. 2003. 60:255–261.

2. Altman DG. Practical Statistics for Medical Research. 1991. 1st ed. London: Chapman and Hall;403–409.
3. Beck JR, Shultz EK. The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch Pathol Lab Med. 1986. 110:13–20.
4. Benedictus G, Dijkhuizen AA, Stelwagen J. Economic losses due to paratuberculosis in dairy cattle. Vet Rec. 1987. 121:142–146.

5. Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 1984. 74:218–262.
6. Colgrove GS, Thoen CO, Blackburn BO, Murphy CD. Paratuberculosis in cattle: a comparison of three serologic tests with results of fecal culture. Vet Microbiol. 1989. 19:183–187.

7. Collins MT, Morgan IR. Economic decision analysis model of a paratuberculosis test and cull program. J Am Vet Med Assoc. 1991. 199:1724–1729.
8. Collins MT, Sockett DC, Ridge S, Cox JC. Evaluation of a commercial enzyme-linked immunosorbent assay for Johne's disease. J Clin Microbiol. 1991. 29:272–276.

9. Collins MT, Wells SJ, Petrini KR, Collins JE, Schultz RD, Whitlock RH. Evaluation of five antibody detection tests for diagnosis of bovine paratuberculosis. Clin Diagn Lab Immunol. 2005. 12:685–692.

10. Cox JC, Drane DP, Jones SL, Ridge S, Milner AR. Development and evaluation of a rapid absorbed enzyme immunoassay test for the diagnosis of Johne's disease in cattle. Aust Vet J. 1991. 68:157–160.

11. Dargatz DA, Byrum BA, Hennager SG, Barber LK, Kopral CA, Wagner BA, Wells SJ. Prevalence of antibodies against Mycobacterium avium subsp. paratuberculosis among beef cow-calf herds. J Am Vet Med Assoc. 2001. 219:497–501.

12. Ferreira R, Fonseca L, Lilenbaum W. Comparison between a commercial and an in-house ELISA for anti-M. avium paratuberculosis antibodies detection in dairy herds in Rio de Janeiro, Brazil. Rev Latinoam Microbiol. 2002. 44:129–134.
13. Gasteiner J, Awad-Masalmeh M, Baumgartner W. Mycobacterium avium subsp. paratuberculosis infection in cattle in Austria, diagnosis with culture, PCR and ELISA. Vet Microbiol. 2000. 77:339–349.

14. Gasteiner J, Wenzl H, Fuchs K, Jark U, Baumgartner W. Serological cross-sectional study of paratuberculosis in cattle in Austria. Zentralbl Veterinarmed B. 1999. 46:457–466.

15. Gilot P, Coene M. Thermostable macromolecular antigens from mycobacteria. Can J Microbiol. 1994. 40:605–611.

16. Gilot P, Misonne MC. Mycobacterium paratuberculosis and Escherichia coli share common antigenic determinants. Vet Microbiol. 1994. 39:353–360.

17. Hill BB, West M, Brock KV. An estimated prevalence of Johne's disease in a subpopulation of Alabama beef cattle. J Vet Diagn Invest. 2003. 15:21–25.

18. Hirst HL, Garry FB, Morley PS, Salman MD, Dinsmore RP, Wagner BA, McSweeney KD, Goodell GM. Seroprevalence of Mycobacterium avium subsp paratuberculosis infection among dairy cows in Colorado and herd-level risk factors for seropositivity. J Am Vet Med Assoc. 2004. 225:97–101.

19. Johnson-Ifearulundu YJ, Kaneene JB, Sprecher DJ, Gardiner JC, Lloyd JW. The effect of subclinical Mycobacterium paratuberculosis infection on days open in Michigan, USA, dairy cows. Prev Vet Med. 2000. 46:171–181.

20. Kim D, Jeon KJ, Kim JT, Shin KS, Shin MK, Chang GH, Kim JK, Kim OS, Jung JY. Prevalence of paratuberculosis of dairy cattle in Kangwon area. Korean J Vet Res. 2002. 42:81–88.
21. Kim JM, Ahn JS, Woo SR, Jo DH, Jo YS, Park JM, Yoon YD, Chang GH. A survey of paratuberculosis by immunological methods in dairy and Korean native cattle. Korean J Vet Res. 1994. 34:93–97.
22. Kim TJ, Kim YS, Kim JC, Yoon WJ, Lee WC, Shin SJ, Chang YF. Studies on molecular biological and immunological diagnosis of Johne's disease. Korean J Vet Res. 1997. 37:349–358.
23. Manning EJ, Collins MT. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Rev Sci Tech. 2001. 20:133–150.

24. Martin SW, Meek AH, Willeberg P. Veterinary Epidemiology: Principles and Methods. 1987. 1st ed. Ames: Iowa State University Press;62–73.
25. Merkal RS, Whipple DL, Sacks JM, Snyder GR. Prevalence of Mycobacterium paratuberculosis in ileocecal lymph nodes of cattle culled in the United States. J Am Vet Med Assoc. 1987. 190:676–680.
26. Meylan M, Nicolet J, Busato A, Burnens A, Martig J. The prevalence of paratuberculosis in the Plateau de Diesse region. Schweiz Arch Tierheilkd. 1995. 137:22–25.
27. Milner AR, Mack WN, Coates KJ, Hill J, Gill I, Sheldrick P. The sensitivity and specificity of a modified ELISA for the diagnosis of Johne's disease from a field trial in cattle. Vet Microbiol. 1990. 25:193–198.

28. Ministry of Agriculture and Forestry. Agricultural and Forestry Statistical Yearbook 2002. 2002. Seoul: Ministry of Agriculture and Forestry;124–125.
29. Ott SL, Wells SJ, Wagner BA. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev Vet Med. 1999. 40:179–192.

30. Pence M, Baldwin C, Black CC 3rd. The seroprevalence of Johne's disease in Georgia beef and dairy cull cattle. J Vet Diagn Invest. 2003. 15:475–477.

31. Pitt DJ, Pinch DS, Janmaat A, Condron RJ. An estimate of specificity for a Johne's disease absorbed ELISA in northern Australian cattle. Aust Vet J. 2002. 80:57–60.

32. Roussel AJ, Libal MC, Whitlock RL, Hairgrove TB, Barling KS, Thompson JA. Prevalence of and risk factors for paratuberculosis in purebred beef cattle. J Am Vet Med Assoc. 2005. 226:773–778.

33. Silva E. Evaluation of an enzyme-linked immunosorbent assay in the diagnosis of bovine tuberculosis. Vet Microbiol. 2001. 78:111–117.

34. Sockett DC, Conrad TA, Thomas CB, Collins MT. Evaluation of four serological tests for bovine paratuberculosis. J Clin Microbiol. 1992. 30:1134–1139.

36. Whittington RJ, Hope AF, Marshall DJ, Taragel CA, Marsh I. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis: IS900 restriction fragment length polymorphism and IS1311 polymorphism analyses of isolates from animals and a human in Australia. J Clin Microbiol. 2000. 38:3240–3248.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


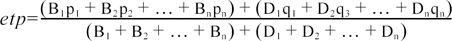




 XML Download
XML Download