Abstract
A bioavailability and pharmacokinetics study of doxycycline was carried out on 30 healthy ostriches after a single intravenous (IV), intramuscular (IM) and oral dose of 15 mg/kg body weight. The plasma doxycycline concentration was determined by HPLC/UV at 0 (pretreatment), 0.08, 0.25, 0.5 1, 2, 4, 6, 8, 12, 24 and 48 h after administration. The plasma concentration-time curves were examined using non-compartmental methods based on the statistical moment theory for only the higher dose. After IV administration, the elimination half-life (t1/2β), mean residence time (MRT), volume of distribution at the steady-state (Vss), volume of distribution (Vdarea) and total body clearance (ClB) were 7.67 ± 0.62 h, 6.68 ± 0.86 h, 0.86 ± 0.16 l/kg, 1.67 ± 0.52 l/kg and 2.51 ± 0.63 ml/min/kg, respectively. After IM and oral dosing, the mean peak plasma concentrations (Cmax) were 1.34 ± 0.33 and 0.30 ± 0.04 µg/ml, respectively, which were achieved at a post-administration time (tmax) of 0.75 ± 0.18, 3.03 ± 0.48 h, respectively. The t1/2β, Vdarea and ClB after IM administration were 25.02 ± 3.98 h, 23.99 ± 3.4 l/kg and 12.14 ± 1.71 ml/min/kg, respectively and 19.25 ± 2.53 h, 61.49 ± 7 l/kg and 40.19 ± 3.79 ml/min/kg after oral administration, respectively. The absolute bioavailability (F) of doxycycline was 5.03 and 17.52% after oral and IM administration, respectively. These results show that the dose data from other animals particularly mammals cannot be extrapolated to ostriches. Therefore, based on these results along with those reported in the literature, further studies on the pharmacokinetic/pharmacodynamic, in vitro minimum inhibitory concentration values and clinical applications of doxycycline in ostriches are required.
Doxycycline is a semi-synthetic bacteriostatic tetracycline and a broad-spectrum antibiotic against Gram-negative and Gram-positive aerobic and anaerobic bacteria, Rickettsiae, Chlamydiae, Mycoplasmas and some protozoa [25,32]. In veterinary medicine, doxycycline is considered to be the corner stone of the treatment for respiratory, urinary and gastrointestinal tract diseases, on account of its pharmacological and pharmacokinetics properties compared with other antimicrobial agents [2,11].
The ostrich, Struthio camelus, is the largest living bird, and has attracted considerable worldwide interest in agriculture. Ostriches provide healthy red meat, excellent quality hides, have reasonable feeding requirements and are hardened to different environmental conditions [32]. However, bacterial infections caused by E. coli, Mannheimia haemolytica, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus viridans, Corynebacterium pyogenes, Chlamydia psittaci, Salmonellae, Colistridium, Proteus, Heamophilus and Mycoplasma species are frequently isolated from ostriches suffering from enteric and respiratory diseases [30]. This can lead to high mortality, particularly in younger ostriches. There is a lack of information on the pharmacokinetics/pharmacodynamics of various drugs in ostriches. Hence, the use of drugs in ostriches including the dosage regimen is based on the data obtained from other animal species [16]. The disposition kinetics of doxycycline has been examined in chicken [3], turkey [29], goat [17,23], calve [20] and pig [5,25] but not in ostriches. Therefore, this study examined the pharmacokinetics and bioavailability of doxycycline in domestic ostriches after a single intravenous (IV), intramuscular (IM) and oral dose.
The working standard powder of doxycycline hydrochloride (hyclate) was obtained from the Arab Pesticide and Veterinary Drug Mfg. (AL-Ramtha, Jordan). The drug was dissolved in water to a final concentration of 150 mg/ml prior to administration.
Thirty healthy ostriches (Struthio camelus), 10-12 months old and weighing 70-90 kg, were used in this study. The ostriches were obtained from a local farm (Albekerat Ostrich Ranch; Amman, Jordan). The animals were monitored for 2 weeks to determine if there were any apparent clinical signs before administering the drug. The animals were housed in an isolated open system pen. The ostriches were given access to water and antibacterial-free food ad libitum.
In a preliminary study, 12 ostriches were divided into three equal groups (4 ostriches/group) in a parallel design. The ostriches in groups 1 to 3 were administered doxycycline (5 mg/kg body weight) IV, IM and orally, respectively. This dose was chosen based on the approved daily dose for goats and sheep [2]. Doxycycline was administered in the right brachial vein and iliotrochanteric muscle for IV and IM administration, respectively, and was given orally through a stomach tube. Food was withheld for 12 h before administering the drug and was given 6 h after. However, the peak level (Cmax) at the dose rate of 5 mg/kg was about one third of other species (1.6 in goat versus 0.5 µg/ml in ostrich) and hence the second dose rate was tripled [2]. In the second trial, doxycycline was given to 30 ostriches at a dose of 15 mg/kg body weight, which had been divided into three equal groups (10 ostriches/group) in a parallel design. The above-mentioned experimental protocol was followed for this trial. A washout period of 14 days was used to ensure complete clearance of the drug from ostriches used in the first trial.
The blood samples (2-3 ml) were collected from the left brachial and cutaneous ulnar veins into heparinized tubes at 0 (pretreatment), 0.08, 0.25, 0.5, 1, 2, 4, 6, 8, 12, 24 and 48 h after administering the drug. The samples were centrifuged directly at ×1,000 g for 5 min. The plasma was then harvested and stored at -20℃ until analysis.
The plasma protein was precipitated by adding 300 µl perchloric acid (16%) to 200 ml of ostrich plasma or a standard sample. The mixture was vortexed for 30 s and centrifuged for 5 min at ×1000 g. The clear supernatant was injected directly into the high performance liquid chromatograph (HPLC).
The plasma concentrations of doxycycline were measured by HPLC according to a slight modification of a previously described method [1]. All the solvents used were of HPLC grade; acetonitrile, methanol and water (Frutarom, UK), and perchloric acid (ApliChem, Germany). The HPLC system consisted of a pump (LC-10A DVP) with UV-vis detector (SPD-10 AVP), auto injector (SIL-10A DVP), solvent degasser (DGV-12 A) and Shimadzu class-VP software (Ver 6.12 SP4) (Shimadzu, Japan). Chromatographic separation was performed using a Purospher Star RP-18e (5 µm, 125 mm ×4.6 mm) column (Merck, Germany) with an isocratic mobile phase acetonitrile: methanol: 0.15% triflouroacetic acid (23 : 25 : 52 v/v/v). The mobile phase was filtered through a 0.45 µm membrane and degassed. The mobile phase was eluted at a flow rate of 1.5 ml/min and detected at a UV wavelength of 347 nm.
A standard calibration curve was prepared by adding 200 µl of doxycycline (1 mg/ml in water) to 800 µl of antibacterial-free ostrich plasma. This was further diluted into antibacterial-free ostrich plasma to produce solutions at concentrations of 0.1, 0.5, 1, 5, 10, 50, 100 and 200 µg/ml. The peak areas were achieved by the measurement of peak area ratios using integration peak program (Class-VP software; Shimadzu, Japan). The HPLC method was validated by assessing the linearity, accuracy, precision, recovery and sensitivity. Two standard calibration curves with 8 doxycycline concentrations (0.1, 0.5, 1, 5, 10, 50, 100 and 200 µg/ml) and 6 replicates of the quality control samples (2, 25 and 150 µg/ml) were prepared and analyzed daily for 3 consecutive days. The calibration curves were linear over the range of 0.10-200 µg/ml. The correlation coefficients of the calibration curves were > 0.9993. The calculated detection limit in the ostrich plasma was 0.05 µg/ml based on a signal-to-noise ratio of 3 : 1, whereas the limit of quantitation was 0.10 µg/ml based on a signal-to-noise ratio of 6 : 1. The analytical recoveries of doxycycline, which were calculated by comparing the peak area ratios for the plasma samples and aqueous samples, ranged from 79.3 to 85.6%. The precision of the inter- and intra-day assay coefficient of variation ranged from 2.4 to 7.8% at concentrations of 2, 25 and 150 µg/ml. The accuracy of the inter- and intra-day assay ranged from 98.1~103.7%.
The pharmacokinetics analysis of the data was performed using non-compartmental analysis based on the statistical moment theory (SMT) according to the method described by Gibaldi and Perrier [13], with the help of a computerized TopFit program [31]. The calculated parameters were: area under plasma concentration-time curve (AUC) using linear trapezoid method; area under the first moment curve (AUMC); mean residence time (MRT), where MRT=AUMC/AUC; volume of distribution (Vdarea), where Vdarea=dose/AUC (β); total body clearance (ClB), where ClB=dose/AUC; apparent volume of distribution at steady state (Vss), where Vss=MRT × CLB; elimination rate (kel) was determined by least-square regression analysis of terminal log-linear portions of the plasma concentration-time profile (kel=2.303 × slop); elimination half-life (t1/2β), where t1/2β=0.639/kel; the maximum concentration (Cmax) and the corresponding peak time (tmax) were determined by inspecting the individual drug plasma concentration-time profiles. The absolute bioavailability (F) was calculated as (AUCnon-IV/AUCIV) ×100.
Statistical analysis was used to evaluate the differences in pharmacokinetics parameters between IV, IM and oral routes. The obtained results were statistically analyzed using student t-test. The differences were considered significant when p < 0.05. All data are expressed as mean ± SE.
In a preliminary study, doxycycline (5 mg/kg body weight) was administered IV, IM and orally to the ostriches in group 1 to 3 (4 ostriches/group), respectively. This dose was based on the approved daily dose for goat and cattle. Fig. 1 shows the mean plasma concentrations-time profile of doxycycline. The concentrations were below the quantifiable concentration (0.1 µg/ml) after 24 h. After a single IV injection of doxycycline at a dose of 5 mg/kg body weight, the mean plasma doxycycline concentration was 29.53 ± 8.52 µg/ml 5 min after injection. After IM and oral administration, the absorption of doxycycline in all ostriches was rapid and measurable at the first sampling time (5 min). The peak plasma concentration of 0.79 ± 0.27 and 0.34 ± 0.02 µg/ml after IM and oral administration was reached at 1 ± 0.35 and 0.52 ± 0.19 h, respectively.
Table 1 and Fig. 2 show the mean plasma concentrations-time profile of doxycycline (15 mg/kg body weight) after IV, IM and oral administration. Table 2 gives the pharmacokinetics parameters. After the single IM and oral administration of doxycycline (15 mg/kg body weight), the plasma drug concentration reached a peak (Cmax) of 1.35 ± 0.33 and 0.30 ± 0.04 µg/ml at 0.75 ± 0.18 and 3.03 ± 0.48 h, respectively. The absolute bioavailability (F) was 5.03 and 17.52% after oral and IM administration, respectively.
The ostrich industry has become economically important. However, to our knowledge, there are no pharmacokinetics reports that have described the mathematical disposition of doxycycline in ostriches through different administration routes. Therefore, clinicians have been obliged to use empirical approaches based on extrapolated doses from other animal species including mammals (sheep, goat and cattle) [16]. This approach may lead less effectiveness, bacterial resistance, as well as increased toxicity and drug residues.
In a preliminary study, the doxycycline plasma concentrations were below the therapeutic concentrations after a single IM and oral dose (5 mg/kg body weight). Therefore, this study examined the pharmacokinetics of doxycycline at a single dose level of 15 mg/kg body weight. The pharmacokinetics of doxycycline (15 mg/kg body weight) after a single IV, IM and oral dose was described adequately using a non-compartmental method, which was based on the SMT. After a single IV dose of doxycycline (15 mg/kg body weight), the t1/2β (7.67 h) was similar to those reported in chickens [3,18] and turkeys [29] but different from those reported in goats [2,17] and calves [27]. The long t1/2β in ostriches suggests that doxycycline is slowly eliminated from the body. The ClB (2.51 ml/min/kg) was low and different from those reported in turkeys [29] and goats [2]. However, these results are similar to those reported in calves [27] and pigs [26]. The long t1/2β calculated in this study indicates a low body clearance value. Doxycycline is highly lipophilic in nature and expected to be distributed more in fatty tissues [28]. The high Vdarea (1.67 l/kg) indicates the rapid distribution of doxycycline in the body. These results are similar to those reported for turkeys [29], pigs [5] and chickens [18]. Whereas, it is lower than those reported in goats [2,17]. The high Vdarea obtained in this study might due to the wide distribution of the drug coupled with its storage in various tissue depots.
Following a single IM dose, the t1/2β (25.02 h) was higher than those reported in healthy East African dwarf goats [23], pigs [25] and non-lactating Egyptian goats [2]. Nevertheless, these results are lower from those reported in infected African dwarf goats [23]. The long t1/2β indicates slow drug release and absorption from the injection site. The other reasons are the drug formula, dosage and other concurrent circumstances associated with the animal's status. In addition, interspecies variation should be considered. The bioavailability (F) of doxycycline after IM administration (17.52%) was lower than those reported in non-lactating Egyptian goats (99.4%) [2]. This might be the result of differences in the physiological and biochemical properties between ostriches and other animal species [6,10].
The most useful PK parameters are the AUC0-24, Cmax and time (T) during which the concentrations exceed a defined PD threshold [19]. On the other hand, the minimum inhibitory concentration (MIC) is the most useful PD parameter. Tetracycline drugs are classified as being concentration-time dependent, where the efficacy is most closely related to the time the plasma concentration exceeds the MIC by 1-5 fold for 40-100% of the inter-dosing interval and ≥ 125 of the AUC/MIC [19]. However, these values have not been validated for doxycycline. Doxycycline reached a peak plasma concentration (Cmax) of 1.35 µg/ml at 0.75 h after the IM injection. These results are in agreement with those reported for goats [2] and pigs [25] but lower than those reported for healthy East African dwarf goats [23]. The mean plasma concentration detected in ostrich plasma after the IM administration of doxycycline (15 mg/kg body weight) was higher than the MICs for Mycoplasma gallisepticum (0.2 µg/ml) [15], Mycoplasma Pneumoniae (< 0.5 µg/ml) [33], Bacteroides fragilis (0.016-0.032) [21], Staphylococcus aureus (0.25 µg/ml) [8], Streptococcus pneumoniae (< 0.4 µg/ml) [4], Streptococcus zooepidemicus (< 1.0 µg/ml) [8], Chlamydia psittaci (0.05-0.2 µg/ml) [9] and porcine bacterial respiratory tract pathogens (Pasteurella multocida, Bordetella bronchiseotica, Actinobacillus pleyropneumoniae and Mycoplasma hyopneumoniae) (0.016-2 µg/ml) [7,24]. However, the doxycycline plasma concentration was lower than the MICs for E. coli (1-4 µg/ml), Pseudomonas aeruginosa (> 64 µg/ml) [22] and Enterococcus fecalis (8-32 µg/ml) [14]. There are no reports on the doxycycline MICs for susceptible microorganisms in ostriches. Therefore, the MICs used for comparison were obtained from other animal species.
After the oral administration of doxycycline at a dose of 15 mg/kg body weight, the t1/2β (19.25 h) was different from those reported in pigs [5,25], chickens [15], horses [12], and calves [20]. The Vdarea was higher and the ClB was lower than those reported in pigs [5]. The bioavailability (F) of doxycycline (5.03 %) was lower than those observed in pigs [5], calves [20], chickens [15] and turkeys [29]. The concentration curves obtained after oral administration indicated a slow absorption rate with a Cmax of 0.30 µg/ml being observed at 3.03 h. The Cmax gained in the study was lower than those observed in pigs [5], calves [20], horses [12] and chickens [18]. On the other hand, the resultant tmax achieved is longer than those reported previously. Anatomical differences between the gastrointestinal tract of ostriches and other animals might contribute to the slow or/and incomplete absorption after oral administration [6]. The Cmax achieved in this study was lower than the MICs for most susceptible bacteria. Nevertheless, doxycycline can be used orally for its local action to treat enteritis associated with susceptible pathogens.
These results show that the dose data from other animals particularly mammals cannot be extrapolated to ostriches. Therefore, further research on a higher dose level, pharmacokinetic/pharmacodynamic, in vitro determination of the MICs and clinical trials will be needed to optimize the efficacy and safe use of doxycycline in ostriches.
Figures and Tables
Fig. 1
Plasma concentration-time profile of doxycycline after an IV, IM and oral dose of 5 mg/kg body weight as determined by HPLC. The values are mean ± SE (n = 4).

Fig. 2
Plasma concentration-time profile of doxycycline after an IV, IM and oral dose of 15 mg/kg body weight as determined by HPLC. The values are mean ± SE (n = 10).

Table 1
Doxycyline plasma concentrations in ostriches after a single IV, IM and oral dose of 15 mg/kg body weight
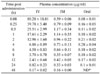
Table 2
Pharmacokinetic parameters of doxycycline in ostriches after a single IV, IM and oral dose

Abbreviations: AUC, area under plasma concentration-time curve; ClB, total body clearance; Cmax, maximum plasma concentration; tmax, time to peak concentration; Vss, the volume of distribution at steady-state; Vdarea, volume of distribution; MRT, mean residence time; t1/2β, elimination half-life; F, absolute bioavailability. Values are mean ± SE (n = 10).
Acknowledgments
This work was supported by Deanship of Research, Jordan University of Science and Technology (Grant No. 163/2005). The authors wish to thank Mr. Ibraheem Albekerat for his generous gift of the animals and location to conduct this research. We wish to thank Mr. Faruq Zghol and Mr. Eyad Hamzeh for technical support in sample analysis.
References
1. Axisa B, Naylor AR, Bell PR, Thompson MM. Simple and reliable method of doxycycline determination in human plasma and biological tissues. J Chromatogr B Biomed Appl. 2000. 744:359–365.

2. Abd El-Aty AM, Goudah A, Zhou HH. Pharmacokinetics of doxycycline after administration as a single intravenous bolus and intramuscular doses to non-lactating Egyptian goats. Pharmacol Res. 2004. 49:487–491.

3. Anadón A, Martínez-Larrañaga MR, Díaz MJ, Bringas P, Fernández MC, Fernández-Cruz ML, Iturbe J, Martinez MA. Pharmacokinetics of doxycycline in broiler chickens. Avian Pathol. 1994. 23:79–90.

4. Aronson AL. Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc. 1980. 176:1061–1068.
5. Baert K, Croubels S, Gasthuys F, De Busser J, Backer P. Pharmacokinetics and oral bioavailability of a doxycycline formulation (doxycycline 75%) in non-fasted young pigs. J Vet Pharmacol Ther. 2000. 23:45–48.

6. Bezuidenhout AJ. The topography of the thoraco-abdominal viscera in the ostrich (Struthio camelus). Onderstepoort J Vet Res. 1986. 53:111–117.
7. Bousquet E, Morvan H, Aitken I, Morgan JH. Comparative in vitro activity of doxycycline and oxytetracycline against porcine respiratory pathogens. Vet Rec. 1997. 141:37–40.

8. Bryant JE, Brown MP, Gronwall RR, Merritt KA. Study of intragastric administration of doxycycline: pharmacokinetics including body fluid, endometrial and minimum inhibitory concentrations. Equine Vet J. 2000. 32:233–238.

9. Butaye P, Ducatelle R, De Backer P, Vermeersch H, Remon JP, Haesebrouck F. In vitro activities of doxycycline and enrofloxacin against European Chlamydia psittaci strains from turkeys. Antimicrob Agents Chemother. 1997. 41:2800–2801.

11. Croubels S, Baert K, De Busser J, De Backer P. Residue study of doxycycline and 4-epidoxycycline in pigs medicated via drinking water. Analyst. 1998. 123:2733–2736.

12. Davis JL, Salmon JH, Papich MG. Pharmacokinetics and tissue distribution of doxycycline after oral administration of single and multiple doses in horses. Am J Vet Res. 2006. 67:310–316.

13. Gibaldi M, Perrier D. Pharmacokinetics. 1982. 2nd ed. New York: Marcel Dekker;409–417.
14. Hoelscher AA, Bahcall JK, Maki JS. In vitro evaluation of the antimicrobial effects of a root canal sealer-antibiotic combination against Enterococcus faecalis. J Endod. 2006. 32:145–147.

15. Ismail MM, El-Kattan YA. Disposition kinetics of doxycycline in chickens naturally infected with Mycoplasma gallisepticum. Br Poult Sci. 2004. 45:550–556.

17. Jha VK, Jayachandran C, Singh MK, Singh SD. Pharmacokinetic data on doxycycline and its distribution in different biological fluids in female goats. Vet Res Commun. 1989. 13:11–16.

18. Laczay P, Semjen G, Lehel J, Nagy G. Pharmacokinetics and bioavailability of doxycycline in fasted and nonfasted broiler chickens. Acta Vet Hung. 2001. 49:31–37.

19. McKellar QA, Sanchez Bruni SF, Jones DG. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J Vet Pharmacol Ther. 2004. 27:503–514.

20. Meijer LA, Ceyssens KG, de Greve BI, de Bruijn W. Pharmacokinetics and bioavailability of doxycycline hyclate after oral administration in calves. Vet Q. 1993. 15:1–5.

21. Mittermayer H, Rotter M, Riezinger F, Thiel W, Stanek G. Susceptibility of the Bacteroides fragilis group to 10 antibiotics. Results of 4 laboratories in Austria. Zentralbl Bakteriol Mikrobiol Hyg (A). 1986. 262:500–511.
22. Moskowitz SM, Foster JM, Emerson J, Burns JL. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J Clin Microbiol. 2004. 42:1915–1922.

23. Ole-Mapenay IM, Mitema ES, Maitho TE. Aspects of pharmacokinetics of doxycycline given to healthy and pneumonic East African dwarf goats by intramuscular injection. Vet Res Commun. 1997. 21:453–462.
24. Pijpers A, Van Klingeren B, Schoevers EJ, Verheijden JHM, Van Miert AS. In vitro activity of five tetracyclines and some other antimicrobial agents against four porcine respiratory tract pathogens. J Vet Pharmacol Ther. 1989. 12:267–276.

25. Prats C, El Korchi G, Giralt M, Cristofol C, Pena J, Zorrilla I, Saborit J, Perez B. PK and PK/PD of doxycycline in drinking water after therapeutic use in pigs. J Vet Pharmacol Ther. 2005. 28:525–530.

26. Riond JL, Riviere JE. Pharmacokinetics and metabolic inertness of doxycycline in young pigs. Am J Vet Res. 1990. 51:1271–1275.
27. Riond JL, Tyczkowska K, Riviere JE. Pharmacokinetics and metabolic inertness of doxycycline in calves with mature or immature rumen function. Am J Vet Res. 1989. 50:1329–1333.
28. Riond JL, Vaden S, Riviere JE. Comparative pharmacokinetics of doxycycline in cats and dogs. J Vet Pharmacol Ther. 1990. 13:415–424.

29. Santos MD, Vermeersch H, Remon JP, Schelkens M, De Backer P, Van Bree HJ, Ducatelle R, Haesebrouck F. Pharmacokinetics and bioavailability of doxycycline in turkeys. J Vet Pharmacol Ther. 1996. 19:274–280.

30. Shane SM. Infectious diseases and parasites of ratites. Vet Clin North Am Food Anim Pract. 1998. 14:455–483.

31. Tanswell P, Koup J. TopFit: a PC-based pharmacokinetic/pharmacodynamic data analysis program. Int J Clin Pharmacol Ther Toxicol. 1993. 31:514–520.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download