Introduction
The enteric nervous system (ENS) is composed of the cell bodies and fibers that are localized between the muscle layers throughout the entire gastrointestinal tract. The ENS integrates the motility, secretion, blood flow and immune response of the digestive tract, independent of the extrinsic autonomic inputs [9,20]. The function of the ENS is to coordinate the complex interactions of the enteric networks, which consist of sensory, inter, motor and secremotor neurons [2,6,9].
Based on localization, the ENS is divided into the myenteric and submucosal plexus. The myenteric plexus is positioned between the outer longitudinal and inner circular muscle layers throughout the digestive tract. Since the myenteric plexus modulates the contraction and relaxation of the smooth muscles [19,20], this plexus is an endogenous source for motor innervation to the muscular layers and secremotor innervation to the mucosa. Similar to other nervous systems, the functions of the myenteric plexus are mediated by various neurochemical substances [2,3,10]. Indeed, substance P (SP) was isolated from the gut [1,3,22] and can be detected in a dense nerve fiber network within the myenteric plexus. SP-containing nerve fibers are an intrinsic contractor of the longitudinal muscle layer [1,3,22]. In addition, calcitonin gene-related peptide (CGRP), which was originally identified as a splicing product of the alternative RNA processing of the calcitonin gene in the rat brain [17], affects a variety of biological activities in the ENS, such as release of gastrointestinal hormone, co-ordination of gastrointestinal motility, excitation of myenteric neurons and vasodilatation.
On the other hand, vitamin-D dependent calcium-binding proteins (CaBPs) were first isolated from the small intestine of the chicken, thus they were presumed to play a primary role in the absorption of dietary calcium [21]. Thereafter, it was discovered that CaBPs transfer calcium across the membranes and regulate free intracellular calcium. Thus, CaBPs act as "buffer/transport" proteins in various cell types. Among them, calbindin D-28k (CB) and calretinin (CR) are observed in ENS neurons characterized by a distinct morphology and specific electrophysiological properties. Furthermore, CaBPs also modulate the release or action of SP and CGRP [4,5,14].
Compared to other species, ruminants have highly specialized stomachs that characterizes these species. The ruminant stomach serves as a large fermentation chamber, and the motility patterns therein maintain vigorous mixing of the ingested food [15]. Neuronal regulation is therefore crucial for the regulation and coordination of region-specific motility in the ruminant stomach. As the exogenous autonomic nervous system is known to play a role in controlling stomach movement, it has been implied that the endogenous ENS may also be involved in regulating the local forestomach functions [7,12]. However, little data are available to elucidate the neurochemical properties of the ENS in ruminants. Thus, we investigated the localization of CB, CR, CGRP and SP immunoreactivity in the myenteric plexus of the goat stomach to characterize the ENS in ruminants.
Materials and Methods
Animal and Tissue Preparation
Twelve goats (Capra hircus, 10-16 months, 15-20 kg B.W.) were used in this study. Goats were obtained from Hallym Animal Center (Korea). All experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals [11].
The animals were anesthetized with ketamine-xylazine mixture and perfused via the common carotid artery with 3 l of 0.9% normal saline followed by 8 l of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The stomach was immediately removed and dissected. The stomach contents were washed by rinsing in ice-cold 0.1M phosphate buffer. The tissues used for cryostat sectioning were stretched and pinned flat on pieces of balsa wood. The tissues were then fixed in 0.1 M phosphate buffer containing 4% paraformaldehyde for 12 h at 4℃ with the mucosal surface facing up. The fixed tissues were washed in 0.1M phosphate buffer and cryoprotected in 0.1M phosphate buffer containing 30% sucrose.
Serial sections of 12 µm were cut using Cryostat (Reichert-Jung, Germany) and mounted on gelatin-coated slides. The sections were stored at -70℃ before processing for immunohistochemistry.
Immunohistochemistry
The sections were pre-incubated in phosphate-buffered saline (PBS) containing 10% normal goat serum (Santa Cruz, USA) for 30 min to reduce nonspecific background staining. The primary antibodies were diluted in 0.1M PBS containing 0.3% Triton X-100 and 2% normal goat serum. The tissues were incubated for 48 h at 4℃ in the solution containing primary antibodies. The following antisera were used at the indicated concentrations: mouse anti-CB (1 : 1,000; Swant, Switzerland), rabbit anti-CR (1 : 1,000; Chemicon, USA), rabbit anti-SP (1 : 2,000; Peninsula, USA) and rabbit anti-CGRP (1 : 2,000; Peninsula, USA).
After the specimens were incubated with the primary antibodies, they were washed three times for 10 min with PBS and incubated for 2 h in buffer solution containing affinity-purified secondary anti-rabbit or -mouse antibodies conjugated to indocarbocyanine (Cy3). Finally, the specimens were washed in PBS and coverslipped with a solution of PBS containing 80% glycerol.
The slide preparations were observed under an Olympus BX51 microscope (Olympus, Japan) attached to an IMT2000 digital camera (iMTechnology, Korea) with the appropriate filters (green filter; exciter filter 513~565 nm; beam filter 580 nm; barrier filter 590 nm). The images of immunoreactivity in the myenteric plexus were captured using Adobe Photoshop version 6.0 software via IMT2000.
Results
In the present study, CB, CR, SP, and CGRP immunoreactivities were observed in the nerve cell bodies or fibers of the stomach myenteric plexuses of the Korean native goat. However, their localizations were distinct in each stomach region.
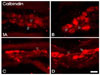
CB immunoreactivity was found in the nerve cell bodies and fibers of the rumen, reticulum and abomasum; however, only CB-immunoreactive (CB-IR) nerve fibers were observed in the omasum (Fig. 1). CB-IR neurons were oval-shaped with generally smooth margins and were classified as Dogiel type II cells based on Dogiel's classification for enteric neurons. The size of the CB-IR neurons was medium to large. The average number of CB-IR cell bodies in the myenteric plexus was 7.8 cells/ganglion.
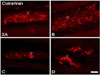
CR-immunoreactive (CR-IR) fibers were found in all subregions of the stomach; in particular, varicosities of CR-IR fibers were dominantly observed in the reticulum (Fig. 2).
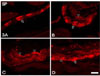
SP-immunoreactive (SP-IR) neurons and fibers were observed in all stomachs, except in the omasum. SP-IR somata were round or oval-shaped (Fig. 3).
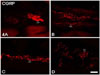
CGRP-immunoreactive (CGRP-IR) fibers were found in all subregions of the stomach, and varicosities of CGRP-IR fibers were prominently observed in the myenteric plexus of goat stomach, compared to CB-, CR-, and SP-IR fibers (Fig. 4).
Discussion
In the present study, we observed CB-, CR-, CGRP- and SP-IR myenteric neurons within the goat stomach. It is known that there are several histologically distinct types of intrinsic neurons in the ENS. These include excitatory and inhibitory motor neurons to the muscle, vasomotor neurons, secremotor neurons, interneurons and sensory neurons. Many investigators have tried to relate these physiological functions to individual cytoarchitecturally defined enteric neurons [2,3,4,5,6,10]. Although the ENS can function independently of the CNS, the latter has an important role in the coordination of the diverse functions of the ENS. The ENS is well connected to the central autonomic neural network in the CNS through both motor and sensory pathways of the sympathetic and the parasympathetic nervous system [2,9]. The parasympathetic motor pathways consist of the vagus and sacral nerves that control motor and secremotor functions. This pathway modulates gastrointestinal responses to stress, eating and behavior. Neurons that carry sensory information to the CNS are known as primary afferent neurons. These neurons are located in the smooth muscle layer and are sensitive to mechanical distention of the gut [6,10].
In the goat stomach, the CB-IR neurons observed in the myenteric plexus were identified as morphological Dogiel type II cells featuring smooth perikarya with long multiple processes. CB-IR cells are known to have the electrophysiological characteristics of after-hyperpolarization (AH), and may be considered to be intrinsic sensory neurons. Moreover, the CB-IR Dogiel type II neurons located in the myenteric plexus of the guinea-pig ileum are known to be cholinergic and to activate the intrinsic primary afferent neurons [4]. In the guinea-pig GI tract, CB-positive neurons function as intrinsic primary afferent neurons or as interneurons. The somata of CB-containing primary afferent neurons in the intestine of several species are located within the myenteric plexus and project to the epithelium [4,9,14]. Based on these previous studies, CB-IR neurons in the myenteric neurons of goat stomach may also be sensory neurons that project to the epithelium. These findings are similar to another study detailing the profiles of in the equine stomach [12]. Therefore, our findings indicate that the localization/functionality of CB in myenteric plexus of the goat may be similar to that of other animals.
In the present study, CR-immunoreactivity was observed in the myenteric ganglia in the goat stomach. In other species, CR is known to be present in the myenteric neurons, and related with sensory function of circular muscle layer and villi. Moreover, CR-IR neurons are identified as small Dogiel type I neurons and sensory intrinsic neurons [2,12,20]. However, CR-IR was only found in the myenteric nerve fibers of goat stomach in this study. These data show that there are differences in the distributions and functions of CR in goat stomach compared to that of other animals.
In a variety of species, SP has been shown to be the major excitatory neurotransmitter of motor neurons in the gastrointestinal tract [3,13,22]. However, a significant difference in the density of SP has been reported in the various regions of the digestive tract; SP-IR neurons were found in the submucosal and myenteric plexuses of small animals, while SP-IR neurons were rarely observed in large animals. Indeed, in the horse [12], SP-immunoreactivity was not observed in the submucosal plexus, and was only weakly detected in the myenteric plexus. In the pig [8], SP-IR neurons and fibers were seen in both plexuses. This discrepancy might be related to the differential ganglionic organization between small and large animals. In the present study, SP-IR myenteric neurons were observed in the goat stomach myenteric plexus. This distribution pattern of SP-IR neurons was similar to other ruminants [13,22]. Since SP affects the contraction of the smooth muscle of the stomach, this peptide stimulates gastric motility and increases the intragastric pressure. These functions may be related to facilitating rumination of ingesta and accelerating gastric transit.
CGRP is a 'marker peptide' of the Dogiel type II cell population in the ganglionated plexuses of the porcine small intestine and has a sensory function [16,19,20,23]. However, we did not observe CGRP-IR myenteric neurons in the goat stomach. Although the function of CGRP-IR neurons in the goat stomach is unclear, our findings indicate that there is species-specific localization of CGRP-IR cells in the myenteric plexus. Similar to CB, the distribution of CGRP-IR fibers indicate that CGRP may be involved in the sensory functions of the stomach, which initiate mixing of the ingesta, regurgitation of the forestomach ingesta, and signal satiety.
In conclusion, the immunohistochemical localization of CB, CR, CGRP and SP in the myenteric plexus of the goat stomach displayed species-specific patterns. These findings suggest that these substances may be directly and/or indirectly related to the gastric functions of the goat stomach.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download