Abstract
Thirty seven cases of bursitis presented to our Veterinary Teaching Hospital from 2001 to 2005. There were 10 adult female buffalos with olecranon bursitis (one had bilateral bursitis) and 26 calves (7 cattle and 19 buffalos, 16 males and 10 females) with presternal bursitis. There were 10 out of 11 cases of olecranon bursitis and 21 out of 26 cases of presternal bursitis with different forms (cystic, proliferative and fibrous) that were removed surgically. The remaining 6 cases, cystic bursitis (olecranon = 1, presternal = 5), were treated by aspiration of their contents and injection of 4% iodine tincture intrabursally. Only 2 cases recovered, 3 cases progressed to fibrosis and required further surgical treatment 2 to 3 weeks later, and 1 case continued to have a cystic lesion. Histopathological examination of tissue specimens from different forms of bursitis revealed that the acquired bursae were generally lined with synovial-like membrane formed from 2-3 cellular layers that covered the connective tissue capsule. The connective tissue capsule differed from one type to another and consisted of fibrous tissues containing numerous small blood vessels, blood capillaries, lymphatics and nerves. There was also evidence for inflammation within the capsule represented by congestion of blood vessels and the presence of perivascular inflammatory cells, mostly mononuclear. In conclusion, surgical treatment was successful and effective for treatment of olecranon and presternal bursitis particularly for the chronic proliferative and fibrous form in cattle and buffalo. The histological structure of the acquired bursae was relatively similar consisting of a synovial-like membrane and a connective tissue capsule with varying degrees of the inflammatory process.
Bursitis, inflammation of bursa, can be true or acquired. True bursitis occurs when inflammation develops in congenital or natural bursa while acquired bursitis is defined as the development of subcutaneous bursa and/or inflammation of that bursa. A common cause of bursitis is direct trauma that gives rise to acute bursitis when it is severe and chronic bursitis when it is mild and repeated [7]. Also, bacterial infection and toxemia have been reported to contribute in the development of bursitis [8]. Davis and Broughton [2] reported on prepatellar bursitis caused by Brucella abortus in cattle.
Bursitis is either acute or chronic. Acute bursitis presents as dry, serous or purulent. Chronic bursitis may follow the acute form and can be cystic, proliferative, fibrous or hemorrhagic. Moreover, chronic bursitis is characterized by accumulation of excessive bursal fluid, thickening of the wall of the bursa by fibrous tissue, extrusions of fibrous bands or septa within the bursal cavity and generalized subcutaneous thickening [7].
Generally, the symptoms associated with bursitis may be acute characterized by painful swelling or may develop into an abscess or chronic form. The later is more common and appears as a fluctuating or fibrous and painless mass [3,8,10].
Treatment of bursitis varies considerably. Acute bursitis is treated by aspiration of the serous fluid and administration of hydrocortisone into the bursa to suppress inflammation. Antibiotics are used in cases with infection. Purulent bursitis is treated like an abscess. However, chronic bursitis is treated by application of absorbent topically like iodine ointment or incision of the bursa with application of an irritant to its interior. Aspiration of the contents and injection of an irritant solution like iodine tincture or 3-5% carbolic acid leads to destruction of the bursal lining followed by granulation, cicatrisation and obliteration of the cavity [7,8].
To date, there is limited information on olecranon and presternal bursitis in cattle and buffalo in the available literature. The present study investigated the outcome of surgical treatment and the histopathological structure of different forms of olecranon and presternal bursitis in cattle and buffalo.
This study was carried out on 10 adult female buffalos with olecranon bursitis (one of them had bilateral olecranon bursitis) (Fig. 1A) and 26 calves (7 cattle and 19 buffalos, 16 males and 10 females) with presternal bursitis. These cases presented to the Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University from 2001 to 2005. Buffalos with olecranon bursitis were adult with an age range of 5-8 years while animals with presternal bursitis were calves with an age range of 1.5-18 months. The size of the bursal swellings ranged from the size of an orange up to the size of a grapefruit.
31 out of 37 bursal swellings (10 olecranon bursitis and 21 presternal bursitis) were treated surgically by en bloc resection and the remaining 6 cases of cystic bursitis (1 olecranon bursitis and 5 presternal bursitis) were treated by aspiration of their contents and injection of 4% iodine tincture intrabursally. Following surgical treatment, tissue specimens were evaluated; they were fixed in 10% neutral buffered formalin, dehydrated in a graded alcohol series, cleared with methyl benzoate and embedded in paraffin wax. Five-micron thick sections were cut and stained with hematoxylin and eosin [1].
Ten out of 11 cases with olecranon bursitis and 21 out of 26 cases of presternal bursitis had surgical removal. Fractious animals were tranquilized using 2% xylazine (0.1 mg/kg b.w.) intramuscularly. During lateral recumbency with the affected limb upper most (in olecranon bursitis) and after aseptic preparation of the operative site, a circular field block around the mass was performed using lidocaine (2%). An elliptical skin incision was performed at the junction between the lateral and caudal aspects of the olecranon bursal swelling or at the ventral aspect of the presternal swelling. The bursal swelling was then removed, excessive skin was excised and the wound was sutured by simple interrupted or interrupted vertical mattress sutures. Surgical treatment of fibrous and proliferative bursitis (Fig. 1B) was easier than for the cystic form as the bursal swellings were circumscribed which facilitated their excision. Surgical treatment was successful for the treatment of olecranon bursitis and in 20 out of 21 cases of presternal bursitis in cattle and buffalo. One buffalo calf, with presternal bursitis, had a postoperative hematoma at the site of surgery which was treated by removal of two stitches and evacuation of the clotted blood and use of an iodine soaked drain for one week after surgery. The remaining cases had complete recovery after surgery.
The remaining 6 cases with cystic bursitis (1-side of the bilateral olecranon bursitis and 5 presternal bursitis) were treated by aspiration of cystic fluid and injection of 4% iodine tincture intrabursally. This technique definitively treated only 2 cases; 3 cases progressed to fibrosis and required further treatment surgically 2 to 3 weeks later and 1 case remained cystic.
Generally, the newly formed bursae that resulted from acquired bursitis appeared to be lined with a synovial-like membrane, lacked a basal lamina and were covered with a connective tissue capsule. Cystic forms of bursitis were lined with 2-3 cellular layers of connective tissue-like cells (Fig. 2A). The underlying connective tissue capsule consisted of dense and loose connective tissue. The dense connective tissue was located directly beneath the cellular lining and formed from mature fibrocytes, fibrous tissue and elastic fibers (Fig. 2B). The loose connective tissue contained few fibrocytes separated by fine connective tissue fibers. The capsule had numerous small blood vessels, blood capillaries and lymph vessels (Fig. 2B). There was also evidence of inflammation represented by congestion of blood vessels and the presence of mononuclear inflammatory cells perivascularly (Fig. 2B). The proliferative form of bursitis was characterized by loss of cellular lining in the presence of only mature connective tissue (Fig. 3A). The underlying capsule was formed by parallel collagenous fibers in between small blood vessels, blood capillaries and nerves (Fig. 3B). Evidence of chronic inflammation was manifested by the presence of lymphocytes perivascularly and perineurally. Moreover, the connective tissue capsule was composed of many colloids projecting into the bursal cavity. These colloids were formed by fibrous tissue with blood capillaries and nerves and a connective tissue core that was lined by mature connective tissue cells with sloughed tissue inside the core. The fibrous form of bursitis was also lined with a fibrous layer and loss of the cellular lining. The underlying capsule was formed by mature fibrocytes, collagenous fibers and numerous small blood vessels and capillaries.
A bursa is a closed sac with cellular lining that resembles the synovial membrane. Bursae interpose between moving parts or at points of unusual pressure such as between bony prominences and tendons [7,8]. According to their position, bursae are classified as subcutaneous, subfascial, subligamentous, submuscular and subtendineous bursae [6]. There are also two types of bursae based on their method of formation; congenital bursae which develop before birth and are located in a constant position and acquired bursae which are formed after birth and generally develop subcutaneously over bony prominences such as the olecranon tuberosity or tuber calcis. Congenital bursae are associated with deep structures. Subcutaneous bursae grow as a result of mechanical effects in postnatal life and might therefore be termed "reactive" or "functional". Movement of skin causes tearing of subcutaneous connective tissue and leads to formation of a gap that becomes filled with fluid with only a few fibrous elements remaining within the bursa when it is finally formed. Accumulated fluid within the bursal cavity becomes encapsulated by fibrous tissue to form this acquired or false bursa [6,8]. It is practical to consider all bursae above the deep fascia as subcutaneous, to consider them all acquired and to classify them as functional or pathologic based on the clinical signs associated with them [7]. Histologically, the walls of the true bursae are lined with a connective tissue membrane that is practically identical to the synovial membrane of joints and may be classified as areolar, fibrous, or adipose as the synovial membrane of joints is classified. Acquired bursae are considered structurally identical to true bursae but their structures depend on the stage of development. Superficial bursae contain synovial-like fluid, but have been shown to be different from joint synovial fluid in terms of viscosity and mucin clot; this suggests differences in hyaluronic acid quantity or quality [7].
In the present study, all cases of olecranon bursitis occurred in adult buffalos around the period of parturition, during the third trimester and continued after parturition. The heavy weight of the animal during pregnancy as well as overgrown claws may be predisposing factors producing constant irritation on the area of the olecranon. By contrast, presternal bursitis occurred in young growing calves and may be attributed to irritation of the presternal area with the manger during feeding. The history of cases indicated that some of these calves were fed through metal constructed or wooden mangers. In addition, the hard ground is considered a predisposing factor resulting in irritation during sternal recumbancy.
Regarding treatment, the surgical excision of bursal swellings had a good outcome. It was successful in 10 out of 11 cases of olecranon bursitis and 21 out of 26 cases of presternal bursitis of different types (cystic, proliferative, fibrous); however cystic bursitis remained a treatment challenge. Consistent with our findings, Piguet et al. [5] reported that surgical excision was successful in 16 out of 17 cases of precarpal bursitis in cattle. Moreover, surgical treatment of four horses with olecranon bursitis had a good outcome. The surgery site healed by primary intention and the owners of the horses were pleased with the cosmetic results. Only one horse showed wound dehiscence at five days after surgery [4]. Treatment of cystic bursitis by aspiration of the cystic fluid and injection of 4% iodine tincture effectively treated only 2 out of 6 cases by regression; there were 3 additional cases that underwent progression to fibrosis and were further treated surgically 2 to 3 weeks later; one of these cases remained cystic. Therefore, skilful surgical excision of the cystic form of bursitis appears to be more effective than conservative treatment with iodine tincture as it was successful in treating all cases of presternal cystic bursitis with no recurrences. All of our cases, olecranon and presternal bursitis, were acquired bursitis as they developed as a result of trauma where the natural bursa is not normally present. The histological structure of these newly formed bursae was relatively similar to the congenital form and the synovial membrane. The latter entities are lined with one or three cell layers of synovial cells without basement membrane. This cellular lining covers a connective tissue layer containing numerous blood capillaries, lymphatics, nerves and a variable amount of adipose cells [9]. To some extent, the newly formed bursae had similar structure. In the cystic form of bursitis, the cellular lining was clearly formed from connective tissue-like cells lacking the basal lamina. In the chronic proliferative form, such lining was lost and replaced by connective tissue formed by mature fibrocytes and fibrous tissue. The underlying connective tissue capsule present differed according to the type of bursitis. For example, the cystic form consisted of dense connective tissue directly found beneath the cellular lining followed by loose connective tissue; while the chronic proliferative type of bursitis was completely composed of dense connective tissue. Generally, the underlying capsule had numerous blood capillaries, lymphatics and nerves. There was also evidence of inflammation represented by congestion of blood vessels and inflammatory cellular infiltration particularly mononuclear cells. The degree of the inflammatory process differed according to the form of bursitis. Some of the cystic forms showed a marked degree of congestion of blood vessels and infiltration with lymphocytes, macrophages and some neutrophils. For the chronic proliferative bursitis, lymphocytes were predominant.
In conclusion, surgical removal of bursitis was effective for the treatment of olecranon and presternal bursitis in cattle and buffalo. En bloc resection provided rapid resolution and may be a more economical approach than conservative treatment. The histological structure of the acquired bursae were similar and consisted of a synovial-like membrane and connective tissue capsule with varying degrees of inflammation.
Figures and Tables
Fig. 1
(A) Olecranon bursitis in a female buffalo. (B) Proliferative form of presternal bursitis in a female buffalo. Note the thin wall and the tissue strands filling the bursal cavity.
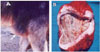
Fig. 2
Histophatological microphotographs of cystic form of presternal bursitis in a male buffalo. (A) Connective tissue-like cells lining the bursal cavity (arrows). (B) Dense connective tissue formed of mature fibrocytes and collagenous fibers. There were also numerous blood capillaries surrounded with inflammatory cells (arrows). H&E strain.
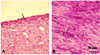
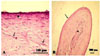
Fig. 3
Histopathological microphotographs of proliferative form of presternal bursitis in a female buffalo. (A) Loss of the cellular lining to more fibrous tissue (asterisk). The underlying connective tissue consisted of mature fibrocytes (arrows) and collagenous fibers. (B) Cross section in connective tissue colloid projected from the wall of bursa into the bursal lumen. Note the connective tissue lining (arrow) and the colloid core (arrowhead). H&E strain.
References
1. Bancroft JD, Stevans A. Theory and Practice of Histological Techniques. 1990. 3rd ed. Edinburgh: Churchill Livingstone;113–305.
2. Davis JM, Broughton SJ. Prepatellar bursitis caused by Brucella abortus. Med J Aust. 1996. 165:460.
3. Dietz O. Diseases of the Horse: a Handbook for Science and Practice. Vol. 2. 1984. Berlin: Karger;14–15.
4. Honnas CM, Schumacher J, McClure SR, Crabill MR, Carter GK, Schmitz DG, Hoffman AG. Treatment of olecranon bursitis in horses: 10 cases (1986-1993). J Am Vet Med Assoc. 1995. 206:1022–1026.
5. Piguet M, Steiner A, Eicher R, Martig J. Surgical treatment of carpal hygroma in cattle: 17 cases (1990-1994). Schweiz Arch Tierheilkd. 1997. 139:210–216.
6. Ottaway CA, Worden AN. Bursae and tendon sheaths of the horse. Vet Rec. 1940. 52:477.
7. Stashak TS. Stashak TS, editor. Lameness. Adams' Lameness in Horses. 1987. 4th ed. Philadelphia: Lea & Febiger;675.
8. Venugopalan A. Essentials of Veterinary Surgery. 1982. 4th ed. New Delhi: Oxford & IBH Publishing;147–165.
9. Weiss L. Histology: Cell and Tissue Biology. 1983. 5th ed. New York: Elsevier Biomedical;525.
10. Wyn-Jones G. Equine Lameness. 1988. Oxford: Blackwell;120–121.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download