Abstract
The ability of a heat-inactivated whole virus from a highly virulent infectious bursal disease virus (hvIBDV) and VP2 protein from hvIBDV expressed in E. coli provided protection against a hvIBDV challenge in specific-pathogen-free (SPF) chickens. Six out of seven chickens that were injected three times with crude VP2 protein developed significant antibody titer against IBDV. However, only four out of the seven chickens survived the hvIBDV challenge. Despite showing low antibody titer profiles, all chickens immunized with the heat-inactivated whole virus also survived the challenged with hvIBDV. However, all of these chickens had bursal atrophy and mild to moderate depletion of lymphocytes. Thus, antibodies raised against IBDV VP2 protein expressed in E. coli and denatured IBDV proteins induced some degree of protection against mortality but not against bursal damage following challenge with hvIBDV.
Infectious bursal disease virus (IBDV) is one of the most important poultry viruses threatening the chicken industry worldwide. The immunosuppressive effect of this virus leads to concurrent secondary infection in the presence of other viruses or bacteria [18]. The most prominent resulting lesions are hemorrhage and necrosis of the bursa of Fabricius, followed by bursal atrophy [20]. The spread of the classical and variant strains of IBDV has not been fully controlled by the introduction of attenuated live and killed variant virus vaccines [18].
Currently, the major problem of IBD is controlling the highly virulent IBDV (hvIBDV) strains which cause more severe damage to the bursa and a higher mortality rate. Due to its markedly different pathogenicity compared to classical strains [22], it can overcome high levels of maternally derived antibodies induced by vaccines protective against previously circulating classical strains of IBDV [31]. The live intermediate plus vaccines are recommended for farms where highly virulent strains are found. However, it has been reported the chickens immunized with intermediate live vaccines develop a certain degree of bursal atrophy and immunosuppression [11]. In addition, the commercially available vaccines have been unable to provide full protection against a hvIBDV challenge [26].
Infectious bursal disease virus VP2 protein is the major antigenic component that encodes for at least two epitopes which induce protective neutralizing antibodies [9]. Hence, numerous studies have been performed to develop an alternative IBDV vaccine by expressing the VP2 protein in various expression systems such as E. coli [3,28], bacteriophages [6], yeast [25], plant [32], fowlpox virus [4], herpesvirus [8], adenovirus [10], Semilik Forest virus [23], baculovirus [30] and plasmid DNA [13]. Vaccination of chickens with these expression products have resulted in variable levels of active or passive protection against mortality and/or bursal damage. However, most of these studies have focused on the use of a standard classical challenge (STC) IBDV as challenge virus to test the recombinant vaccines.
The E. coli expression system is known to be the fastest, easiest as well as an inexpensive technique for expression of usable amounts of recombinant protein. Recombinant VP2 expressed in E. coli reacted with a range of monoclonal antibodies [2,14]. However, it has been reported that VP2 protein expressed in E. coli is not suitable for the production of subunit vaccine [3,14]. However, a recent study reported that VP2 protein expressed in E. coli can induce up to 100% protection against both mortality and bursal damage caused by STC IBDV [28]. Hence, the efficacy of VP2 protein expressed in E. coli for protection against virulent IBDV has not been fully resolved. In addition, the importance of VP2 protein expressed in E. coli for protection against a hvIBDV challenge remains unclear. In this study, we studied the efficacy of heat-inactivated whole virus, of hvIBDV strain UPM97/61, and its recombinant VP2 protein expressed in E. coli for protection against a hvIBDV challenge in specific-pathogen-free (SPF) chickens.
The VP2 gene used in this study was obtained from the local hvIBDV strain UPM97/61 [12]. The amplification, cloning and sequencing of the VP2 gene was performed using methods previously described [7]. The putative complete open reading of the VP2 gene (1351 bp) from position 132 to 1483 followed the nucleotide numbering system of Bayliss et al. [5]; it was cloned in the TOPO cloning vector (Invitrogen, USA) following the methods recommended by the manufacturer.
The TOPO cloning vector containing the VP2 gene was subcloned into a pRSET vector version A (Invitrogen, USA) using BglII and EcoRI restriction enzyme sites (MBI Fermentas, USA). The ligation mixtures were transformed into competent E. coli BL21-SI. Five clones were picked at random from LB plates and screened for positive clones as well as the orientation of the inserted VP2 gene using PCR methods. The recombinant plasmid was confirmed by restriction digestion and sequencing of flanking regions.
A positive clone was selected for expression in LBON medium containing 100 µg/ml ampicillin. Expression was induced by adding sterile NaCl to 0.3 M and incubation was continued for 5 h before harvesting. The expression of VP2 protein was confirmed by Western blot analysis. Briefly, the cells were centrifuged at 3500 g for 10 min and the pellet was resuspended in 1X loading buffer. The mixtures were subjected to 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) [17] using the vertical slab gel Mini Protean II (Bio-Rad, USA). The gels were then stained with Coomasie brilliant blue. For Western blot analysis, the separated proteins from SDS-PAGE were transferred to the polyvinylidene difluoride (PVDF) membrane (Immun-Blot, Bio-Rad, USA). The membranes were incubated with rabbit IBDV polyclonal anti-sera (1 : 80,000) for 45 min at room temperature with agitation. The membranes were then washed in washing buffer and followed by the incubation of alkaline phosphatase-conjugated antibody to rabbit IgG (1 : 5000) (KPL, USA). Finally, the blots were developed with BCIP/NBT color reagents according to manufacturer's instructions (KPL, USA).
Prior to the production of large-scale recombinant protein, a small scale optimization was carried out to estimate the optimum conditions for expression. The parameters being studied were the concentration of NaCl (0.2 M, 0.3 M, 0.4 M), the cell incubation temperature (27℃, 30℃, 33℃) and the incubation time (1 h, 2 h, 3 h). The production of large-scale recombinant protein was carried out in one flask under optimal conditions. The VP2 recombinant protein was purified from the crude protein using the ProBond Purification System (Invitrogen, USA) as described by the manufacturer's manual. The quantification of the total protein concentration was determined using Bio-Rad Protein Assay (Bio-Rad, USA).
A total of 43 one-week-old SPF chicks were randomly allocated to six groups. The first two groups were uninfected and used as a negative control; the third to sixth groups were inoculated intramuscularly with 150 µg of crude E. coli protein, 150 µg of crude VP2 protein, 50 µg of purified VP2 protein and heat-inactivated 106 EID50 of UPM97/61 virus. The inoculums were mixed vigorously with equal amounts of Freund's complete adjuvant (Sigma, USA) during the first immunization while Freund's incomplete adjuvant (Sigma, USA) was used for subsequent immunizations.
The immunizations were boosted twice at two week intervals. Blood for serum was collected from each group at day 0, 7, 14, 21, 28 and 35 post-immunization. One week after the last immunization (day 35), all the chickens except chickens in the second group were challenged orally with 104.8 EID50 of UPM97/61 [12]. Chickens in the second group served as unchallenged negative controls and were used for scoring bursal lesions. All of the chickens were monitored daily for mortality, and the bursas were collected for scoring lesions.
On day eight post-challenge all of the surviving chickens were sacrificed and the bursa to body weight ratios were determined. The bursal tissues from both dead and surviving chickens were also processed for histopathological examination using a lesion scoring system previously described [12]. The lesions were defined as: 0 (normal), 1 (mild), 2 (mild to moderate), 3 (moderate), 4 (moderate to severe) and 5 (severe).
An enzyme linked immunosorbent assay (ELISA; IDEXX, USA) was used to assay the antibody levels produced to IBDV using the methods recommended by the manufacturer. The antibody titers were calculated based on the following calculation, log10 titer = 1/09(log10 SP) + 3.36. Samples with antibody titer greater than log10 2.60, which is equal to 396, were considered positive.
The pRSET vector expressed the VP2 protein together with polyhistidine taq at the C-terminus. It was estimated that the VP2 protein constituted about 3.8% of the total expressed protein. Based on the Western blot analysis an expected protein band of VP2 protein was detected with the approximate size of 50 kDa (Fig. 1). Solubility testing also indicated that most of the protein was expressed in soluble form (data not shown).
Optimal conditions for expression vary depending on the specific protein being expressed. The main factors affecting protein expression are the concentration of NaCl, temperature and the incubation time. Protein expression occurred at 27℃, 30℃ and 33℃ without a significant difference in the amount of protein being expressed based on the results of the Western blot analysis (data not shown).
There was a reduced amount of recombinant protein produced in 0.1M NaCl. At 0.2 M to 0.4 M of NaCl, the amount of protein produced was not significantly different (Fig. 2). There was no difference at incubation times of 2 h, 3 h and 4 h (data not shown). In general, an incubation temperature of 30℃ for 3 h and with 0.3 M of NaCl provided the optimal condition for expression of VP2 protein in the BL21-SI cells.
The mean IBDV antibody titers for each group were measured by ELISA from day 0 to 35 days post-immunization (Table 1). All of the antibody titers were below significant levels on day 0 indicating that the birds were not infected with IBDV. The uninfected control birds had no significant detectable antibody titers throughout the experiment. Six out of seven chickens that were injected with 150 µg of crude VP2 protein developed antibody titers against IBDV by day 35 (Table 1 and 2). Compared to the other groups, only chickens immunized with crude VP2 recombinant protein had antibody titers more than log10 3.0. As shown in Table 2, two out of seven chickens in this group had the highest antibody titers which were log10 3.04 and log10 3.21.
As shown in Table 1, even though the mean antibody titers at day 21 and 28 were higher than at day 14, the titers were considered low or negative for IBDV. In order to confirm that the production of antibodies was not due to the interference of E. coli proteins present in the cell lysate, a group of chickens injected with E. coli protein only was included in the experiment. Results showed that the E. coli protein did not stimulate the production of antibody above a significant level (Table 1). None of the chickens had a positive antibody titer prior to challenge by day 35 post immunization (Table 2). In the case of chickens immunized with purified VP2 protein, production of antibodies throughout the study was below significant antibody titers except for one chicken that was positive for IBDV on day 21 and 28 (Table 1). By day 35 post immunization, two of the chickens developed a positive antibody titer measuring log10 2.63 and log10 2.73 (Table 2). However, chickens immunized with heat-inactivated UPM97/61 virus had different antibody titer profiles compared to the other groups. The antibody titers of two to three chickens in this group were positive for IBDV at day 14, 21 and 28 (Table 1). By day 35 post immunization, however, the antibody titers remained low ranging from log10 2.62 to log10 2.80 (Table 2).
All of the chickens in the groups that were challenged with hvIBDV showed signs of inactivity and depression. Except for the chickens that were immunized with the heat-inactivated virus, chickens in the other groups began to die on the third day after being challenged. All of the chickens immunized with heat-inactivated IBDV survived the challenge; however, four out of the seven (57%) chickens that were immunized with 150 µg of crude VP2 protein remain alive throughout the experiment (Table 1). Only 10% of the chickens that were not immunized survived the challenge while two out of seven chickens (28%) survived the challenge following immunization with E. coli protein.
Post-mortem analysis confirmed that all of the dead chickens had obvious hvIBDV infection characterized by moderate to severe bursal edema, hemorrhage and/or necrosis. Even though all of the chickens immunized with heat-inactivated virus survived the challenge, they had a reduced bursa to body weight ratio (Table 3). No observable significant lesions (score 0) were present in the control birds throughout the experiment. The majority of chickens that survived the challenge showed the presence of mild (score 1) and mild to moderate (score 2) bursal lymphoid depletion (Table 3). However, histopathological examination of the bursa from dead chickens showed evidence of moderate to severe (score 4) and severe (score 5) lymphoid necrosis and depletion both in the medulla and cortex of the follicles of the bursal tissues (data not shown). Severe edema with heavy infiltration of heterophils was also observed in the interstitial area.
The immunogenicity and protective efficacy of non-replicating IBDV vaccines with heat-inactivated whole virus and VP2 protein expressed in E. coli were evaluated in SPF chickens. The VP2 protein was successfully expressed in soluble form using a pRSET vector following induction with NaCl in E. coli host cell strain BL21-SI cells. Compared to uninoculated and E. coli protein inoculated chickens, chickens inoculated with VP2 protein elicited specific antibody titers which were detectable by ELISA. This suggests that the VP2 protein was processed and presented to the humoral immune system. These results support previous studies that E. coli expressed VP2 protein is able to induce the synthesis of antibodies [2,3]. However, the findings also showed that the antibodies induced by E. coli expressed VP2 protein reacted specifically with denatured viral protein, but less well than with intact virus.
There are not many reports on the use of the hvIBDV as a challenge virus to test recombinant vaccine. It has been only recently shown that recombinant herpesvirus expressing VP2 protects chickens against hvIBDV induced illness and mortality; with 67% of chickens protected against gross bursal lesions [29]. In another study, it was reported that only 50% of the chickens vaccinated with VP2 DNA vaccine were protected against mortality but not against bursal atrophy following challenge with a Korean hvIBDV [15]. In this study, we demonstrated that chickens immunized three times with heat-inactivated whole virus and E. coli expressed VP2 protein developed antibodies against IBDV. However, only four out of seven chickens, that had the highest antibody titer after immunized with VP2 protein, were protected against the hvIBDV challenge. However, all of the other chickens that had generated a low to moderate antibody titer, ranging from log10 2.53 to log10 2.71, succumbed to the hvIBDV challenge.
By contrast, all of the chickens immunized with heat-inactivated whole virus, despite low antibody titers ranging from log10 2.00 to log10 2.80, were protected against the challenge with hvIBDV. The reason for this finding is not clear; these findings probably represent the presence of epitopes of other IBDV proteins such as VP3 and/or an additional VP2 epitope that is not present in the E. coli expressed VP2 protein; these molecules might have played a role in inducing protective immunity. It has been shown earlier that chickens vaccinated with a VP2, VP3 and VP4 based vaccine were better protected against virulent IBDV compared to chickens vaccinated with VP2 vaccine alone [15,19]. In addition, it has been predicted that the VP2 protein of a hvIBDV strain HK46 has at least three potential glycosylation sites [6]. Since, VP2 protein expressed in E. coli is poorly glycosylated, some crucial epitopes especially conformational epitopes, were not formed and presented to the chicken immune system. Furthermore, it has been previously shown that induction of neutralizing antibodies against IBDV infection is dependent on high conformation of epitopes located on the VP2 protein [9]. Hence, it is likely that the antibody titer obtained from chickens immunized with VP2 protein was directed against linear but not conformational epitopes due to improper folding during the expression of VP2 in E. coli.
The inability of chickens immunized with purified VP2 protein to confer high antibody titers, associated with protection against a hvIBDV challenge, is not understood. The low level of protection may be due to the purification steps which might have altered or removed the presence of epitope(s) that are crucial for antibody production. Alternatively, E. coli expressed VP2 protein may have failed to form epitopes essential for the induction of neutralizing antibody; a previous study has shown that chickens immunized with purified VP2 protein, expressed in baculovirus, were protected against a STC IBDV challenge [24]. As shown in Table 1, it appears that the inoculation of E. coli protein alone does influence the outcome of the challenge study. The reason for this is unknown; it is likely that E. coli protein induces the activation of innate immunity. The role of bacterial proteins, as well as DNA, in stimulating innate immunity especially macrophage activity has recently been reviewed [1,16].
As shown in Table 1, the heat-inactivated UPM97/61 IBDV conferred 100% protection of chickens challenged with hvIBDV in terms of mortality; this was observed even though the average antibody titer was low or below a significant level. However, it is interesting to note that all of the chickens that survived the challenge following immunization with VP2 protein and inactivated whole virus have a significantly reduced bursa to body weight ratio compared to the unchallenged chickens. Therefore, this study demonstrated that the presence of high antibody titers, against the appropriate conformational epitopes that are present in live virus, is essential to induce protection against bursal atrophy. A recent study suggests that the antigen conformation of IBDV vaccine influences both the quality and quantity of antibody induced by the VP2 protein [21]. Another alternative explanation is that the inactivated whole virus vaccine might have induced other components of the immune response in particular cell-mediated immune (CMI) responses. Recently, it has been reported that under normal conditions, IBDV stimulates a protective antibody response; however, in the absence of antibody, CMI responses alone especially T cell responses are adequate for protecting birds against virulent IBDV [27]. In another recent study, it was reported that CMI responses, rather than humoral immune responses, appeared to contribute to the protection of chickens against hvIBDV infection [15].
In conclusion, the results of this study suggest that inappropriate expression and folding of IBDV proteins particularly VP2 protein that occurs in the E. coli expression system or in heat denatured IBDV proteins, failed to induce antibody responses that were associated with protection against hvIBDV induced bursal damage. The protective efficacy of the studied proteins in inducing protection against STC IBDV infection is also not known. However, the humoral antibody responses provided varying degrees of protection against mortality following a challenge with hvIBDV. Further studies on the importance of both humoral antibodies as well as CMI responses following inoculation with VP2 protein expressed E. coli in inducing protection against STC IBDV as well as hvIBDV infections are currently underway.
Figures and Tables
Fig. 1
Western blot analysis of VP2 protein. Lane 1, standard protein marker; lane 2, BL21-SI host cells only and lane 3, cell lysate sample of 3 h induced recombinant plasmid. Arrow indicates the evidence of ~50 kDa VP2 protein detected by rabbit polyclonal antibody against IBDV.

Fig. 2
Optimization of NaCl concentration for expression of VP2 protein in the E. coli strain BL21-SI. Lane 1, standard protein marker; lane 2, 0.1 M NaCl; lane 3, 0.2 M NaCl; lane 4, 0.3 M NaCl and lane 5, 0.4 M NaCl. Arrow indicates that the highest expression of VP2 protein was achieved with 0.3 M NaCl.
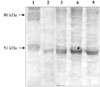
Table 1
Antibody titers, number of chickens with positive antibody titers and percentage of chickens protected against a challenge with highly virulent IBDV

*The ELISA was carried out using IDEXX Infectious bursal disease antibody test kit at serum dilution of 1 : 500. The antibody titers were calculated based on the following calculation, log10 titer = 1/09(log10 SP) + 3.36. Samples with antibody titer greater than log10 2.60 which is equal to 396 were considered positive. The titers were expressed as mean log10 ± SD.
Acknowledgments
This work was funded by IRPA grant number 01-02-04-0297 from the Ministry of Science, Technology and Innovations, Government of Malaysia. The authors would like to thank Dr. Egbert Mundt, Federal Research Centre for Viral Diseases of Animals, Germany for providing the antibodies against VP2 protein.
References
1. Aucouturier J, Dupuis L, Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine. 2001. 19:2666–2672.

2. Azad AA, Fahey KJ, Barrett SA, Erny KM, Hudson PJ. Expression in Escherichia coli of cDNA fragments encoding the gene for the host-protective antigen of infectious bursal disease virus. Virology. 1986. 149:190–198.

3. Azad AA, McKern NM, Macreadie IG, Failla P, Heine HG, Chapman A, Ward CW, Fahey KJ. Physicochemical and immunological characterization of recombinant host-protective antigen (VP2) of infectious bursal disease virus. Vaccine. 1991. 9:715–722.

4. Bayliss CD, Peters RW, Cook JKA, Reece RL, Howes K, Binns MM, Boursnell MEG. A recombinant fowlpox virus that expresses the VP2 antigen of infectious bursal disease virus induces protection against mortality caused by the virus. Arch Virol. 1991. 120:193–205.

5. Bayliss CD, Spies U, Shaw K, Peters RW, Papageorgiou A, Muller H, Boursnell MGE. A comparison of the sequences of segment A of four infectious bursal disease virus strains and identification of a variable region in VP2. J Gen Virol. 1990. 71:1303–1312.

6. Cao YC, Shi QC, Ma JY, Xie QM, Bi YZ. Vaccination against very virulent infectious bursal disease virus using recombinant T4 bacteriophage displaying viral protein VP2. Acta Biochim Biophys Sin (Shanghai). 2005. 37:657–664.

7. Chong LK, Omar AR, Yusoff K, Hair-Bejo M, Aini I. Nucleotide sequence and phylogenetic analysis of a segment A of a highly virulent strain of infectious bursal disease virus. Acta Virol. 2001. 45:217–226.
8. Darteil R, Bublot M, Laplace E, Bouquet JF, Audonnet JC, Riviere M. Herpesvirus of turkey recombinant viruses expressing infectious bursal disease virus (IBDV) VP2 immunogen induce protection against an IBDV virulent challenge in chickens. Virology. 1995. 211:481–490.

9. Fahey KJ, Erny K, Crooks J. A conformational immunogen on VP2 of infectious bursal disease virus that induces virus-neutralizing antibodies that passively protect chickens. J Gen Virol. 1989. 70:1473–1481.

10. Francois A, Chevalier C, Delmas B, Eterradossi N, Toquin D, Rivallan G, Langlois P. Avian adenovirus CELO recombinants expressing VP2 of infectious bursal disease virus induce protection against bursal disease in chickens. Vaccine. 2004. 22:2351–2360.

11. Giambrone JJ, Closser J. Efficacy of live vaccines against serologic subtypes of infectious bursal disease virus. Avian Dis. 1990. 34:7–11.

12. Hoque MM, Omar AR, Chong LK, Hair-Bejo M, Aini I. Pathogenicity of Ssp1-positive infectious bursal disease virus and molecular characterization of the VP2 hypervariable region. Avian Pathol. 2001. 30:369–380.

13. Hulse DJ, Romero CH. Partial protection against infectious bursal disease virus through DNA-mediated vaccination with the VP2 capsid protein and chicken IL-2 genes. Vaccine. 2004. 22:1249–1259.

14. Jagadish MN, Staton VJ, Hudson PJ, Azad AA. Birnavirus precursor polyprotein is processed in Escherichia coli by its own virus-encoded polypeptide. J Virol. 1988. 62:1084–1087.

15. Kim SJ, Sung HW, Han JH, Jackwood D, Kwon HM. Protection against very virulent infectious bursal disease virus in chickens immunized with DNA vaccines. Vet Microbiol. 2004. 101:39–51.

16. Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002. 20:709–760.

17. Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970. 227:680–685.

19. Li J, Huang Y, Liang X, Lu M, Li L, Yu L, Deng R. Plasmid DNA encoding antigens of infectious bursal disease viruses induce protective immune responses in chickens: factors influencing efficacy. Virus Res. 2003. 98:63–74.

20. Lukert PD, Saif YM. Calnek BW, Barnes JH, Beard CW, McDougald LR, Saif YM, editors. Infectious bursal disease of poultry. Diseases of Poultry. 1997. 10th ed. Ames: Iowa State University Press;721–738.
21. Martinez-Torrecuadrada JL, Saubi N, Pages-Mante A, Caston JR, Espuna E, Casal JI. Structure-dependent efficacy of infectious bursal disease virus (IBDV) recombinant vaccines. Vaccine. 2003. 21:3342–3350.

22. Oppling V, Muller H, Becht H. Heterogeneity of the antigenic site responsible for the induction of neutralizing antibodies in infectious bursal disease virus. Arch Virol. 1991. 119:211–223.

23. Phenix KV, Wark K, Luke CJ, Skinner MA, Smyth JA, Mawhinney KA, Todd D. Recombinant Semliki Forest virus vector exhibits potential for avian virus vaccine development. Vaccine. 2001. 19:3116–3123.

24. Pitcovski J, Di-Castro D, Shaaltiel Y, Azriel A, Gutter B, Yarkoni E, Michael A, Krispel S, Levi BZ. Insect cell-derived VP2 of infectious bursal disease virus confers protection against the disease in chickens. Avian Dis. 1996. 40:753–761.

25. Pitcovski J, Gutter B, Gallili G, Goldway M, Perelman B, Gross G, Krispel S, Barbakov M, Michael A. Development and large-scale use of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine. 2003. 21:4736–4743.

26. Rautenschlein S, Kraemer CH, Vanmarcke J, Montiel E. Protective efficacy of intermediate and intermediate plus infectious bursal disease virus (IBDV) vaccines against very virulent IBDV in commercial broilers. Avian Dis. 2005. 49:231–237.

27. Rautenschlein S, Yeh HY, Sharma JM. The role of T cells in protection by an inactivated infectious bursal disease virus vaccine. Vet Immunol Immunopathol. 2002. 89:159–167.

28. Rong J, Cheng T, Liu X, Jiang T, Gu H, Zou G. Development of recombinant VP2 vaccine for the prevention of infectious bursal disease of chickens. Vaccine. 2005. 23:4844–4851.

29. Tsukamoto K, Sato T, Saito S, Tanimura N, Hamazaki N, Mase M, Yamaguchi S. Dual-viral vector approach induced strong and long-lasting protective immunity against very virulent infectious bursal disease virus. Virology. 2000. 269:257–267.

30. Vakharia VN, Snyder DB, Lutticken D, Mengel-Whereat SA, Savage PK, Edwards GH, Goodwin MA. Active and passive protection against variant and classic infectious bursal disease virus strains induced by baculovirus-expressed structural proteins. Vaccine. 1994. 12:452–456.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download