Introduction
Ultraviolet (UV) irradiation, in particular the middle wavelength range (UVB, 280-320 nm), has been clearly shown to be the primary cause of non-melanoma skin cancer [25]. Studies have indicated that UV irradiation induces skin cancer primarily by its DNA-damaging properties and also its capacity to suppress the immune system. The mechanism of UV-induced immune suppression is not completely understood. Evidence is accumulating, however, that UVB-induced DNA damage represents a key step in the initiation of immune suppression [2], although other mechanisms, such as the photoisomerization of urocanic acid [20], free radical formation [7], and signal transduction-mediated activation of transcription factors [8,23], may play a role as well. Following UV exposure, DNA forms cyclobutane pyrimidine dimers (CPDs) and (6-4) photoproducts [6]. UV irradiation-induced CPD formation, in particular, leads to the release of immunosuppressive cytokines, such as IL-10, from keratinocytes [19]. Furthermore, in >90% of UV-induced human skin cancers, CPDs can be localized within the cell cycle regulatory gene p53 [5,31], which also acts as a tumor suppressor gene, suggesting a significant role of UV-induced DNA damage in photocarcinogenesis. Hence, UV irradiation-induced DNA damage is the crucial event for suppression of the immune system, which leads to skin cancer development.
Recent studies in mice with topically applied isoflavonoids derived from the red clover, Trifolium pratense, have shown that these phytochemicals may protect effectively against UV radiation-induced skin damage. Particular interest has centered on equol ([S]-4'7-dihydroxyisoflavane), an isoflavonoid metabolite produced from the dietary isoflavone daidzein by the gut microflora in mammals. Equol has been found to protect not only against UV radiation-induced cutaneous inflammation, observed as the sunburn reaction, but also against photoimmune suppression, which is evident as a defective contact hypersensitivity (CHS) reaction initiated by the UV-irradiated skin [29]. The immunoprotective mechanism of topically applied equol involves the inactivation of the downstream actions of cis-urocanic acid, a major UV-induced immunosuppressive photoproduct produced in the skin [29]. Photoimmune suppression is a prerequisite for the promotion of UV radiation-initiated tumors, and topically applied equol has been demonstrated to have anti-photocarcinogenic properties in mice [28], thus the photoprotective effects of equol offer the opportunity to identify critical pathways linking the UV-induced immune defect with the development of skin cancer. In addition, equol has the capacity to stimulate the induction of metallothionein (MT), which is one of the endogenous cutaneous antioxidants that have been identified as relevant for protection against oxidative photodamage [27].
Because the state of DNA damage has also been correlated with suppression of the immune system and photocarcinogenesis, it was of interest to study the possible DNA damage protection effects of topical equol, when applied to the skin before and after moderate short-term acute SSUV exposure. We hypothesize that the observed anti-immunosuppressive and anti-carcinogenic effects of topically applied equol [28,29], are associated with equol's protection of DNA against damage following SSUV exposure. In this study, we report the effect of topical application of equol to the skin of the hairless mouse prior to, or immediately after, SSUV exposure on the reduction and removal of CPDs by the immunohistochemical staining method.
Materials and Methods
Animals
Age-matched inbred female hairless albino (Skh:HR-1) mice aged 8-10 weeks were used in this study (Veterinary Breeding Colony University of Sydney, Australia). They were housed in wire-topped plastic boxes on compressed paper bedding (Boral, Australia), and maintained at 25℃ with 12 h light (GEC F40G0 gold light, which does not emit any UV), alternating with 12 h dark. They were fed standard laboratory mouse pellets (Norco Stockfeeds, Australia) and tap water ad libitum. All procedures for using animals were approved by the University of Sydney Animal Ethics Committee and complied with the State Animal Research Act 1985.
UV Irradiation
SSUV radiation was carried out as described previously [29]. One group of mice was exposed to SSUV on the dorsum, with the daily dose providing 0.167 J/cm2, which is approximately one minimum erythemal dose (1MED) for this mouse each day, for three consecutive days (3 × 1 MED). The other group was exposed to a single SSUV dose providing 0.501 J/cm2 of UVB, which is approximately equal to 3MED (1 × 3 MED of SSUV exposure).
Topical lotion
Equol was purchased from Sigma-Aldrich, Australia. The compound was dissolved in DMSO, then diluted to the required concentration in an innocuous oil-in-water cosmetic lotion (base lotion) as described previously [28,29], without color, perfume, or preservative, so that the final lotion contained 20 mM of equol and 0.5% (vol/vol) DMSO. Aliquots of 0.2 ml were applied to the dorsum of mice and spread with a gloved finger; the mice were held on their cage top while the lotion was absorbed into the skin.
Protocol of study
Two different protocols of treatment were conducted to observe the effect of the SSUV exposure regime on the CPD appearance and the time course of their removal. These treatment groups were: (i) 3 × 1 MED and (ii) 1 × 3 MED of SSUV exposure. No topical application of equol or base lotion was used in this protocol. Three different protocols of treatment were conducted to assess the effect of equol on the reduction and removal of CPD following SSUV exposure. These treatment groups were: 1 × 3 MED of SSUV exposure plus equol, which was applied (i) immediately after SSUV exposure for 5 days; (ii) for 7 days prior to SSUV exposure and then continued for 5 days immediately after SSUV exposure and (iii) 1 × 3 MED of SSUV exposure plus base lotion, which was applied immediately after SSUV exposure for 5 days.
Immunohistochemical staining for the detection of CPDs
The immunonostaining procedure for CPD detection was carried out as previously described [22] with a slight modification. The H3 antibody, a monoclonal antibody specific for cyclobutane thymine dimers, was purchased from TNO Nutrition and Food Research (Netherlands).
Collection of skin samples: Two mice from each treatment group were killed 30 min after 1 × 3 MED and 3 × 1 MED of SSUV exposure and at 1, 24, 48, 72 and 168 h after exposure. Mid-dorsal skins of mice were excised, fixed in Histochoice (Amresco, USA) for 4 h, processed overnight with a formalin-based automated methodology, and paraffin-embedded. Subsequently, tissue sections were cut at 5 mm onto poly-l-lysine coated slides (Sigma-Aldrich, Australia).
Immunohistochemical staining: The tissue sections were deparaffinized through xylol and graded ethanol solutions to 70%. Endogenous peroxidase was quenched by incubation in 0.3% (vol/vol) hydrogen peroxide (Riedel-deHaen, Germany) in methanol. Citric acid buffer (pH 6.0) and microwave treatment were used for antigen retrieval. To denature DNA in situ, tissue sections were then incubated in 50% ethanol for 5 min, 0.02 N HCl in 30% ethanol for 2 min, 0.05 N HCl for 5 min, for 7 min in freshly prepared 0.07 N NaOH in 70% ethanol, and then treated with 30 µg proteinase K in 1 ml Tris calcium chloride (Sigma, USA), at 37℃ for 10 min. To absorb non-specific mouse tissue reactivity, the tissue sections were incubated in specific mouse IgG (1 : 25 dilution) (MOM kit; Vector, USA) prior to incubation with the H3 antibody.
H3 antibody was applied onto each tissue section (1 : 75 dilution) and incubated overnight at 4℃. Subsequently, the tissue sections were incubated at room temperature for 40 min with biotinylated anti-mouse IgG (1 : 250 dilution; Vector, USA). Finally, the immunoreactions were visualized using avidin-biotin peroxidase complexes (Vectastain ABC kit; Vector, USA), and the peroxidase reaction was developed in 3,3'-diaminobenzidine chromogen (Dako, USA). Tissue sections were counterstained with haematoxylin, followed by dehydration through graded ethanol solutions to 100%, and then mounted in DPX. Non-immune mouse serum in PBS containing 10% FCS was used as an isotype control (Mouse IgG; ICN Biomedicals, Australia).
Analysis of the presence of CPDs: The number of positive staining cells was counted using a program in a Leica Q500 MC image processing and analysis system (Leica Microsystem, Switzerland). Positively stained CPDs (brown color) were identified on the displayed image and also recorded photographically with the Adobe Photoshop program (Adobe Photoshop 5.5). Excessive boiling in citric acid buffer severely damaged the dermal cutaneous compartment. Therefore, only the number of thymine dimers in the epidermal layer of the skin were counted. A minimum of 20 randomly selected fields per section from each mouse were analyzed.
Results
Under our experimental conditions, in which SSUV was used as a source of radiation, positively stained CPDs were found in the nuclei and in the cytoplasm of epidermal and dermal layers of the skin. Only those CPDs observed in the epidermal cutaneous compartment were selected for the study detailed in this paper.
Effect of the UV exposure regime on the CPD appearance and the time course of their removal Fig. 1 shows that the peak of CPD appearance occurred at 1 hour after a single dose of 3 MED SSUV exposure (1 × 3 MED of SSUV exposure). Subsequently, at 24 and 48 h post-SSUV exposure, there was apparent removal of CPDs, down to 47% and 79% respectively (p < 0.01 and p < 0.001 respectively). These dimers then completely disappeared from the epidermal cells by 168 h after irradiation. In contrast, when 3 irradiations with 1 MED of SSUV daily (3 × 1 MED of SSUV exposure) was applied, the peak of CPD appearance was established at 48 h following the first SSUV exposure (p < 0.05). Subsequently, by 72 h post-SSUV irradiation, the appearance of these dimers markedly decreased (p < 0.01), revealing 42% removal of CPDs. In this model of radiation, the CPD response after the first exposure to 1 MED was significantly less than after 3 MED of SSUV (p < 0.01). Moreover, the CPDs induced by subsequent SSUV had an additive effect, and after the third SSUV exposure resulted in approximately the same number of dimers as after 1 × 3 MED of SSUV exposure. These CPDs were mostly removed, but some remained present at 168 h.
Effect of equol on the reduction and the removal of CPDs following SSUV exposure
Topical application of 20 mM equol lotion was observed to protect against SSUV-induced CPDs, when it was applied for 7 days prior to SSUV exposure and 5 days following (b/a) SSUV exposure (Fig. 2). Equol that was applied both before and after SSUV significantly reduced the number of CPDs. This reduction was evident immediately after SSUV exposure and at 1 and 24 h afterwards (p < 0.01; revealing 54%, 50%, and 26% reduction of CPDs respectively), compared to the control group (SSUV only) at the same time points, and was still significant at 48 h post-SSUV irradiation (p < 0.05). By 72 h after SSUV exposure, there was no significant difference in the number of CPDs in the control group, or the groups treated with equol (p > 0.05). These dimers had completely disappeared from the epidermal cells by 168 h after irradiation in all groups (p > 0.05). On the other hand, when 20 mM equol was applied for 5 consecutive days after SSUV exposure (a), there was no significant difference in the number of CPDs immediately after SSUV irradiation, or at 1 h afterwards, compared to the control group (p > 0.05), even though it appears that there were fewer CPDs (Fig. 2). As the dimers were removed at 24 and 48 h after SSUV exposure, the number remained less than in the control group, revealing a significant reduction of 23% and 42% in CPDs, respectively (p < 0.05). By 72 h after SSUV exposure there was no significant difference in the number of CPDs. However, the rate of CPD removal in the equol-treated mice was not different from the rate in control mice. Thus, equol protected significantly from CPD formation only if applied prior to SSUV exposure.
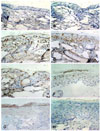
The removal of CPDs following a single dose of 3 MED SSUV (1 × 3 MED of SSUV exposure) is shown histologically in Fig. 3 (a-h). Chronologically, immediately after and at 1 h after SSUV exposure, most of the CPDs were found in the basal layer of the epidermis. By 24 and 48 h post-SSUV irradiation, CPDs were found in the upper layer of the epidermis and only a few were detected in the basal layer of the epidermis. Subsequently, at 72 h post-SSUV exposure, CPDs were found in the keratinocyte layer, but had completely disappeared by 7 days post-SSUV irradiation. UV-induced CPD formation increases the chance of inheritable changes in p53 genes. This chance is greater in tissues with proliferating cells than in tissues with resting cells [5,31]. These findings support our results, demonstrating that CPDs were initially found in the basal layer of the epidermis, in which the cells are normally actively proliferating.
Discussion
Exposure to ultraviolet B radiation (UVB), demonstrated to be the main waveband that is responsible for both UVinduced immunosuppression [15] and photocarcinogenesis [13], results mainly in the production of CPDs and (6-4) photoproducts. These comprise about 95% of the UVB-induced DNA lesions [10], seem to be pre-mutagenic in mammalian cells [4], and play a crucial role in both photocarcinogenesis and photoimmunosuppression [5,14].
Under our experimental conditions, CPDs were found intracellularly, both in epidermal and dermal cells following SSUV irradiation. This finding was consistent with a previous immunofluorescence staining study that demonstrated that, following UVB exposure (8 J/m2), CPDs were present in both the nuclei and cytoplasm of the dermal and epidermal cells [24]. We observed that the peak of CPD appearance and the time course of their removal depended on the UV exposure regime. The peak of CPD appearance was within 1 h after 1 × 3 MED of SSUV exposure. Repeated irradiation (3 × 1 MED of SSUV exposure) resulted in the peak of CPD appearance at 48 h after the first SSUV exposure. However, there were fewer CPDs at 1 h after the first 3 × 1 MED of SSUV exposure than after 1 × 3 MED of SSUV irradiation, indicating a UV dose responsiveness. A linear dose-dependent induction of CPDs in hairless mouse epidermis following UVB irradiation has been demonstrated in a previous study [26]. Following 1 × 3 MED of SSUV exposure, there was apparent removal of CPDs (approximately 47%) at 24 h and they completely disappeared from the epidermal cells by 168 h after irradiation, consistent with previously described studies [21,26]. In contrast, after repeated SSUV exposure, there was obvious removal of CPDs (approximately 42%) after 72 h, which was 24 h after the final irradiation, but some remained for up to 168 h after SSUV exposure. Thus, this finding indicates that following repeated irradiation, the removal rate of CPDs is slower than after a single dose of UV exposure. This retardation of CPD repair may be due to the effects of UV on DNA repair enzymes, e.g. nucleotide excision repair, as described in an in vitro study [12].
By using a single dose of SSUV (1 × 3 MED of SSUV exposure), we found that equol, applied before SSUV exposure, protected against SSUV-induced DNA damage by reducing the number of CPDs substantially at 0.5 h and significantly at 24-48 h. This finding suggests that equol may act as a mild UVB sunscreen, since it has a UV absorption peak in the UVB waveband at 281 nm [9]. Our unpublished data also demonstrated that repeated applications of equol caused residual equol to accumulate in the skin and provided a mild sunscreening effect against exposure of SSUV administered 24 h later. Mouse studies have shown that sunscreens can inhibit DNA photodamage [16,30] and p53 mutation [1]. Moreover, since dimer formation is important in erythema [17], and sensitivity to erythema/edema in mice has been associated with the inability to carry out transcription-coupled repair of thymine dimer lesions [3], we suggest that the mild sunscreening effect of residual equol during chronic application may have endowed protection from UV-induced skin inflammation in mice (erythema-associated oedema) [29], mediated via reduced CPD formation. Moreover, some slight evidence suggested that topically applied equol after SSUV exposure might encourage CPD removal at 24-48 h. Despite apparently reducing the number of CPDs, equol applied topically post-SSUV irradiation did not appear to increase the rate of dimer removal at other time points (0.5-168 h). However, our results need substantiating in the future, perhaps by using more sensitive techniques for CPD quantitation, such as flow cytometry or alkaline gel electrophoresis.
Transformation of urocanic acid (UCA) from its trans to its cis isomer in the stratum corneum has been shown to exhibit properties of immunosuppression [11]. Moreover, there is also evidence that UCA may be involved in DNA damage through its ability to photosensitize the formation of pyrimidine dimers in vitro [18]. This indicates that the DNA and UCA-mediated photoimmunosuppression effects might be linked. We established that the immunoprotective properties of equol against acute UV exposure were achieved by inactivation of cis-UCA [29]. Therefore, we speculate that inactivation of cis-UCA by equol might impede UCA's ability to photosensitize the formation of CPDs, partially accounting for the reduced number of CPDs formed in SSUV-irradiated mice treated with equol.
In conclusion, the results of this study have shown that, whereas topical application of equol provided significant protection against formation of CPDs when it was applied prior to SSUV exposure, application of equol following SSUV irradiation did not result in significant reduction in CPD formation. This study also suggested that equol present on the skin prior to SSUV irradiation can contribute to the protection we report against SSUV-induced erythema-associated oedema, immunosuppression [29], and skin cancer [28], by acting as a sunscreen and thus inhibiting DNA photodamage.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download