Despite the advances in diagnosis, surgery, antimicrobial therapy, and intensive care support, the mortality rate in humans with severe peritonitis remains high [
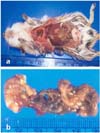
4]. In this study, the macroscopic findings of induced experimental peritonitis in Mongolian gerbils were similar to those of clinical peritonitis observed in humans. The parietal peritoneum was thickened and adhered to the abdominal organs. The entire gastrointestinal tract was covered with a thin fibrous film and the mesentery was thickened and contracted. The use of the Mongolian gerbil as an animal model for peritonitis might contribute to determining the mechanisms of clinical peritonitis in humans, and may also help in establishing the therapeutic treatment by screening drugs for preventing peritonitis.
In this study, an animal model, the Mongolian gerbil, was used to develop experimental peritonitis using an oral dose of indomethacin, a nonsteroidal anti-inflammatory drug (NSAID), which is widely used as an analgesic, anti-inflammatory and antipyretic. The main concern for these drugs is the frequency and severity of their digestive side effects involving the entire gastrointestinal tract. The fecal calprotectin is altered in 44% [
17] of patients taking NSAID, and scintigrams suggest the presence of small intestinal inflammation in 42% of cases. However, when fecal excretion of [
111In]-labeled leukocytes was used as a measure of inflammation, up to 67% [
13] of patients were found to have enteropathy. Similarly, in a post-mortem study, intestinal lesions were detected by enteroscopy [
10] in 66% of patients, and 13.5% of subjects had nonspecific intestinal ulceration [
1]. The intestinal lesions caused by NSAID can mimic inflammatory bowel disease [
9] and might lead to chronic bleeding, protein loss, strictures, diverticulitis, appendicitis, internal fistulas, and a relapse of inflammatory bowel disease. The pathogenesis of NSAID enteropathy is complex and is not completely understood. There are two main pathogenic mechanisms. The first involves specific biochemical damage to the mitochondria [
15] with uncoupling of the oxidative phosphorylation reaction. This increases the intestinal permeability and the release of calcium into the cytosol, which in turn causes secondary biochemical damage [
2]. The second, and more controversial mechanism involves the inhibition of cyclooxygenase (COX)-1 and/or COX-2 and its products (prostaglandins). However, some studies have reported that COX inhibition is not involved [
3], while another study suggested that COX inhibition is the key event [
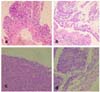
16]. In this study, indomethacin induced the dose-dependent and time-dependent formation of macroscopically apparent peritoneal lesions in Mongolian gerbils. Grossly, these animals showed dark red and sticky material in the jejunum, ileum and an enlarged colon. Histopathologically, the jejunum of these animals had mucosal erosions and hemorrhagic lesions. Histopathological scores of peritoneal inflammation were significantly increased by the administration of indomethacin (
p < 0.05). Also, peritonitis was revealed by the significant changes of WBC and platelet count (
p < 0.05). The results of biochemical analysis were evidenced the hepatic and renal damages in group 3 and 4. The cause of the peritonitis in this study suggests that it might be due to the increased permeability of the jejunum caused by the administration of indomethacin. Both total protein albumin level and histological findings could suggest it.
This study found an effective and simple method of inducing peritonitis by oral dosing of indomethacin at a rate of 30 mg/kg body weight using 5-week old Mongolian gerbils. The resulting peritonitis that developed can be used as an effective tool for investigating the potential therapeutic compounds aimed at preventing peritoneal damage during peritonitis and provide insight into the pathophysiology of peritonitis.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download