Introduction
Caveolin has been shown to be involved in many signal transduction mechanisms, including phosphorylation by protein kinases, and signaling by G-proteins [8]. Caveolin is also known as a cholesterol-binding membrane protein involved in cellular cholesterol transport and homeostasis [3,5]. In addition, caveolin seems to be related to caveolae formation, where it plays an important role in the specific localization of proteins and other components involved in signal transduction [4]. Three isotypes of the protein have been identified: caveolin-1, -2, and -3; both caveolin-1 and caveolin-2 are abundantly expressed in a variety of tissues including brain [6], and heart [15]. Because signal transduction and lipid metabolism are central to the function of nervous tissues, recent studies have focused on the expression of caveolins in the nervous system. Normal brain tissue has been examined [7], as well as brain from Alzheimer's disease patients [5], as well as the spinal cord tissue from experimental autoimmune encephalomyelitis [12].
The retina is a remote organ of the central nervous system, and consists of various cell types, including neuronal cells and glial cells [13]. Caveolin-1 plays an important role in the normal physiology of the retina [1], and in pathological conditions including a prion disease model [9]. However, little is known about the normal expression of caveolin in the retina of the rat. The aim of the present study was to examine and to localize the expression of each of the caveolins in the rat retina.
Materials and Methods
Animals
Male Sprague Dawley rats (200-250 g) were obtained from Chungang Animal Laboratory (Korea). The aniamals were housed in cages in a standard barrier facility, and maintained on a 12-h light: 12-h dark cycle at 23℃. The research was conducted in accordance with the internationally accepted principles for laboratory animal use and care as found in the NIH guidelines (USA).
Tissue sampling
To collect samples, six rats were deeply anesthetized with chloral hydrate (Fluka, Switzerland) (375 mg/kg body weight, intra-peritoneal injection), and the eyes were enucleated. The anterior segments of both eyes were removed from each rat, and the retina of one eye was separated for Western blot analysis. The eyecup of the second eye was fixed by immersion in 10% formalin in 0.1 M phosphate buffered saline (PBS), pH 7.4, for 24 h before paraffin embedding.
Antibodies
The antibodies used in this study included mouse monoclonal anti-caveolin-1 (IgG1, clone 2297), and anti-caveolin-2 (IgG1, clone 65), all obtained from BD Biosciences (USA). Mouse monoclonal anti-beta-actin was obtained from Sigma (USA). The specificity of each monoclonal antibody to caveolin-1, or -2 has been well characterized; no cross-reactivity between antibodies has been detected [10,11,14].
Western blot analysis
The retinas and hearts from rats were separately homogenized in lysis buffer (40 mM Tris, 120 mM NaCl, 0.1% Nonidet 40, 2 mM Na3VO4, 1 mM PMSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin) and then centrifuged to remove any insoluble material. Samples were mixed with loading buffer and electrophoresed under denaturing conditions by 12% SDS-polyacrylamide gel electrophoresis. The proteins were then electroblotted in transfer buffer to nitrocellulose membranes (Schleicher & Schuell, USA) for 2 h at 100 V. Residual binding sites on the membranes were blocked by incubation with 5% non-fat milk in Tris-buffered saline (TBS; 10 mM Tris-HCl, pH 7.4, and 150 mM NaCl) for 1 h. Membranes were then incubated for 1 h with mouse antibody against either caveolin-1, or caveolin-2. The blots were washed three times in TBS containing 0.1% Tween-20, and then probed with horseradish peroxidase-conjugated anti-mouse IgG (Vector, USA) for 1 h. The blots were developed using enhanced chemiluminescence reagents (Amersham, USA), according to the manufacturer's instructions. Heart tissue was used as a positive control for caveolin-1 and -2 [15]. After imaging, the membranes were stripped and reprobed using monoclonal anti-beta-actin antibody as the primary antibody (Sigma, USA).
Immunohistochemistry
Paraffin sections (5 µm) of rat eyecups were deparaffinized and pretreated with citrate buffer (0.01 M, pH 6.0) in a microwave oven for 2 min. After hydration, the sections were treated with 0.3% hydrogen peroxide in distilled water for 20 min to block endogenous peroxidase activity. After three washes in PBS, the sections were exposed to 10% normal horse serum and then incubated for overnight at 4℃ with mouse monoclonal antibodies against either caveolin-1, or caveolin-2 (all at 1 : 200 dilution). After three further washes, the sections were incubated with biotinylated second antibody, followed by formation of avidin-biotin peroxidase complexes using the Elite kit (Vector, USA). The peroxidase reaction was developed with a diaminobenzidine substrate (Vector, USA). Before mounting, the sections were counterstained with hematoxylin. In the negative control, no immunostaining was seen in sections where primary antiserum was omitted (Fig. 2B).
Results
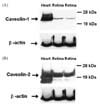
Western blot analysis of caveolin-1 and -2 in rat retina
Western blotting revealed the presence of caveolin-1 (Fig. 1A), and caveolin-2 (Fig. 1B) in the retinas of adult rats. Caveolin-1 immunoreactivity was detected in a band with an approximate molecular weight of 22 kDa. The same band was also observed in rat heart extracts, and observed to run in parallel, confirming the specificity of the antibody for caveolin-1 (Fig. 1A). For caveolin-2, there were two bands typically detected in retinas, a major band of approximately 20 kDa, and a minor band of approximately 18 kDa (Fig. 1B).
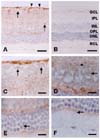
Immunohistochemical localization of caveolin-1 and -2 in rat retina
Expression of caveolin-1 was detected in the internal limiting layer and optic nerve fiber layer (Fig. 2A). In the ganglion cell layer, caveolin-1 was weakly localized to the cell membranes and nuclei of ganglion cells (Fig. 2C). Caveolin-1 was diffusely expressed in the plexiform layer, which contained bipolar axons, ganglion cell dendrites, and amacrine cell processes (Fig. 2C). In the inner nuclear layer, caveolin-1 immunoreactivity was weakly detected in the cellular membranes and nuclei of amacrine cells, and bipolar cells (Fig. 2D). Caveolin-1 staining was diffuse in the outer plexiform layer, which contains axonal ends of photoreceptor cells, dendritic processes of the bipolar cells and processes of the horizontal cells (Fig. 2E). In the outer nuclear layer, weak caveolin-1 immunoreactivity was seen in photoreceptor cells, and in the external limiting membrane, but little was detected in inner segments of rods and cones (Fig. 2F).
The immunostaining pattern of caveolin-2 in the retina was largely the same as that of caveolin-1, but was much weaker (Fig. 3A). Caveolin-2 immunostaining was however, intensely detected in vessels (Fig. 3A). Also, caveolin-2 was diffusely stained in inner plexiform layer (Fig. 3B), inner nuclear layer (Fig. 3C), and outer plexiform layer (Fig. 3D). Caveolin-2 was immunostained in the processes of glial cells (Fig. 3A, arrowhead) and of identical Muller cells (Fig. 3C, arrow). Caveolin-2 immunoreactivity was very limited in retinal neuronal cells including the ganglion cells, amacrine cells, bipolar cells, horizontal cells, and photoreceptor cells (Fig. 3B-D).
Discussion
This study confirms that caveolin-1 and -2 are expressed in the eye, specifically in the retina, of rats. Based on the functional role of caveolins, these molecules may be assumed to play an important role in the cholesterol homeostasis of the retina. An ultrastructural study has shown that caveolin-1 is localized in the synaptic ribbons in rod and cone photoreceptors of bovine retina, but not on synaptic vesicles or in presynaptic plasma membranes [7].
Using light microscopy, we found that the two isoforms of caveolin could be identified in the rat retina, and that caveolin-1 was localized in a number of retinal cell layers. Although some studies have described the presence of caveolin-1 in the rod and cone layer in tissues examined by transmission electron microscopy [2], here, little immunostaining was found in that layer. In addition to the rod and cone layer, caveolin-1 was diffusely immunostained in the ganglion cell layer, inner plexiform layer, and inner nuclear layer, suggesting that caveolin-1 has activity in these cell layers.
The present study has also confirmed that caveolin-2 is typically localized in the majority of vessels in the retina, and in a few process-bearing cells, but that the expression of caveolin-2 is quite low compared with that of caveolin-1. This finding suggests that the function of caveolin-2 may be unique, although its sequence is similar to that of caveolin-1 [10,11].
In summary, we have found that all caveolins were diffusely immunostained in the inner and outer plexiform layers of rat retina. These layers contain a variety of neuronal and glial cells, and have numerous synaptic junctions. This finding, together with other evidence from this and other studies, suggests that the caveolin-1 and -2, individually or in combination, may be involved in synaptic signal transduction in the retina. The determination of their precise role in the retina will require further study.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download