Abstract
Porcine reproductive and respiratory syndrome (PRRS) is one of the most important swine diseases worldwide. In the present study, a new virulent strain of PRRS virus (PRRSV), GDsg, was isolated in Guangdong province, China, and caused high fever, high morbidity, and high mortality in sows and piglets. The genome of this new strain was 15,413 nucleotides (nt) long, and comparative analysis revealed that GDsg shared 82.4% to 94% identity with type 2 PRRSV strains, but only 61.5% identity with type 1 PRRSV Lelystad virus strain. Phylogenetic analysis indicated that type 2 PRRSV isolates include five subgenotypes (I, II, III, IV, and V), which are represented by NADC30, VR-2332, GM2, CH-1a, and HuN4, respectively. Moreover, GDsg belongs to a newly emerging type 2 PRRSV subgenotype III. More interestingly, the newly isolated GDsg strain has multiple discontinuous nt deletions, 131 (19 + 18 + 94) at position 1404–1540 and a 107 nt insertion in the NSP2 region. Most importantly, the GDsg strain was identified as a virus recombined between low pathogenic field strain QYYZ and vaccine strain JXA1-P80. In conclusion, a new independent subgenotype and recombinant PRRSV strain has emerged in China and could be a new threat to the swine industry of China.
Porcine reproductive and respiratory syndrome (PRRS) includes reproductive diseases in sows including abortion, mummified fetus, and stillbirth, and respiratory system diseases in pigs of all ages [1]. Since it emerged in 1980s, the disease has spread to most pig farms in the world and has led to huge economic losses [9].
The PRRS pathogen is the porcine reproductive and respiratory syndrome virus (PRRSV), which is a positive-strand RNA virus belonging to the Arteriviridae family [21]. The length of the PRRSV genome is about 15 kb and includes at least eight open reading frames (ORF): ORF1a, ORF1b, and ORFs 2–7 [7]. ORF1a and ORF1b encode the polyproteins pp1a and pp1b, respectively, which are further cleaved into nonstructural proteins (NSPs): NSP1α, NSP1β, and NSP2–12 [4]. ORFs 2–7 encode structural proteins GP2, GP3–5, M, and N [3]. The PRRSV can be classified into two genotypes: type 1 (European type: representative strain Lelystad virus, LV) and type2 (North American type: representative strain VR-2332) [24]. The genomes of both genotypes have only 60% nucleotide (nt) homology, but both types led to similar clinical symptoms and pathological changes [10].
The first PRRSV was isolated in China in 1996, and, since that time, the virus has undergone extensive evolution, and in 2006, there was an atypical PRRS outbreak in South China, which was characterized by high fever, high morbidity, and high mortality [1723]. Further study indicated that the outbreak was caused by a highly pathogenic PRRSV (HP-PRRSV) with a characteristic 30 amino acid (aa) deletion in its NSP2 region [36]. In 2015, some NADC30-like strains were isolated in Northeast and Central China, and phylogenetic analyses indicated that they had high identity similarity with the moderately virulent NADC30 strains isolated in America in 2008 and belonged to a new subgroup; some NADC30 like strains can result in high mortality in swine [3335]. Interestingly, at the end of 2015, we identified a novel PRRSV strain, GDsg, which had been isolated from sick pigs at a PRRS-outbreak farm in China. GDsg was observed to be different from all of the aforementioned strains and exhibited large-scale nt deletions, insertions, and evidence of recombination. As this new strain could spread widely, GDsg is considered a potential threat to the swine industry. Herein, we report on the results of our characterization of GDsg.
In 2015, lung samples were collected from suspected PRRSV positive sick pigs infected with PRRSV in Guangdong province, South China. The lung samples were homogenized and centrifuged and the supernatants were used for virus isolation. All samples were collected according to the animal ethical regulation of National Engineering Center for Swine Breeding Industry (NECSBI 2015-16).
Virus isolation was performed in porcine alveolar macrophages (PAMs), which were maintained in RPMI-1640 at 37℃ containing 10% fetal bovine serum (Thermo, USA), 100 mg/mL penicillin, and 100 units/mL of streptomycin. Initially, the PAMs were seeded in 6-well cell culture plates (Corning, USA) and incubated with supernatants from the homogenized lung samples for 1 h. The supernatants were then discarded, RPMI-1640 medium added to the 6-well culture plates, and the PAMs maintained at 37℃ with 5% CO2. The cultured cells and supernatants were harvested when a cytopathic effect appeared in 70% of the cells. The recovered virus was designated as strain GDsg. The GDsg strain was passaged twice in PAMs and purified by plaque assay. Viral cultures of the purified virus were used for genomic sequence analysis.
To determine the full genomic sequence of GDsg, primers were designed based on the reference PRRSV sequences available through the National Center for Biotechnology Information (NCBI, USA). The primers used for GDsg genome sequencing are described in Table 1.
Total RNA was extracted by using TRIzol reagent (Life Technologies, USA) according to the manufacturer's instructions. Reverse transcription (RT) was performed in a total volume of 20 µL containing 10.5 µL total RNA, 4 µL 5× RT buffer, 2 µL deoxynucleoside triphosphate mixture (10 mM), 1 µL 9-mer random primers (50 pM), 2 µL RT M-MLV (Takara Biotechnology, China), and 0.5 µL RNase inhibitor (40 U/µL). The reactants were mixed gently, placed in a water bath at 42℃ for 1 h, then incubated on ice for 2 min. The polymerase chain reaction (PCR) was conducted by using PrimeSTAR HS DNA Polymerase (Takara Biotechnology).
The PCR products were purified by using the Wizard SV Gel and PCR Clean-Up system (Promega, USA), and then cloned into pEASY Simple Blunt vector (TransGen Tech, China). Plasmids were submitted to BGI (China) for sequencing, and the complete PRRSV genome sequence was obtained by using the SeqMan program within DNAstar 7.0 software (DNAstar, USA). The complete genome sequence was named GDsg and was submitted to GenBank under accession number KX621003.
The nt and deduced aa sequences were aligned by using the MegAlign program in DNAstar 7.0 software and used to determine sequence homology. A phylogenetic tree was constructed by using MEGA software (ver. 5.2) [28] and the neighbor-joining method; bootstrap values were calculated for 1,000 replicates for alignment with multiple sequences of representative PRRSV sequences available in GenBank (Table 2).
To detect probable recombination events, the genomic sequence was scanned for possible recombination event indicators by using SimPlot (ver. 3.5.1) software according to the methods described previously [19]. The parent virus sequences were for QYYZ and JXA1-P80 and the query was the GDsg genomic sequence. A window of 200 bp and a step size of 20 bp were applied.
A PRRS outbreak happened in Shaoguan city in Guangdong province, China on 30 December 2015, resulting in 70 sows abortion and 3 sows death. The abortion rate among the pregnant sows was 11.43% (120/1,050). The sows experiencing abortion and death had high body temperatures (40.5℃–41.5℃). The mortality rates among suckling and nursery piglets were approximately 60% and 20%, respectively. The PCR diagnostic test results indicated that the samples from the dead sows were PRRSV positive, but negative for porcine circovirus2 (PCV2), porcine encephalomyocarditis virus (PEMCV), porcine pseudorabies virus (PRV), classical swine fever (CSFV), porcine parvovirus (PPV), porcine epidemic diarrhea virus (PEDV), and transmissible gastroenteritis virus (TGEV). Lung samples from the 3 dead sows were collected and a PRRSV field strain, named GDsg, was isolated after culturing with PAMs. The GDsg strain was passaged in PAMs twice and the viral cultures of the third passage were used for genomic sequence analysis. Total viral RNA was extracted from the viral cultures and 13 genomic fragments were amplified by performing RT-PCR with the relevant primers. The PCR products were cloned into the pEASY Simple Blunt vector (TransGen Tech) for sequencing. The whole genome sequence was obtained by using the sequence splicing method in SeqMan software. The GDsg genomic sequence was determined to be 15,413-nt long, including a 189-nt 5′UTR, a 151-nt 3′UTR, and eight ORF. The genomic sequence of GDsg was compared with other PRRSV reference strains (Table 3). Genetic and evolutionary analyses showed that GDsg shared a high nt homology with QYYZ (94%) and GM2 (93%), but a low nt similarity with North American reference strain VR-2332 (86.4%). In addition, it had 89.5% and 90% genomic similarities with the Chinese low pathogenic strain CH-1a and the Chinese HP-PRRSV strain JXA1, respectively. GDsg shared a much lower nt similarity with NADC30 (83.3%) and the recently isolated in China NADC30-like strain CHsx1401 (82.4%). Interestingly, it had a very low genomic homology with the European type LV strain (61.5%). Different ORFs had different nt sequence identity, ranging from 78.9% to 98.2% when compared to type 2 strains. The lowest sequence identities were in ORF1a, while the highest identities were in ORF6.
The 189-nt 5′UTR of GDsg had a 92.6% to 100% nt identity with the type 2 reference strains, however, only a 59% nt identity with the type 1 reference strain LV. The 3′UTR of GDsg had 151 nt and was followed by a 26-nt poly(A) tail. The sequence alignment indicated that the 3′UTR of GDsg displayed 86.8% to 96.7% identity with other type 2 strains and 68.2% identity with the type 1 strain LV.
The GDsg ORF1a encoded a 2496 aa pp1a which was further cleaved into several NSPs: NSP1a, NSP1b, and NSP2 to NSP8. NSP2 of GDsg showed low nt (72.4%–88.6%) and aa (66.9%–86.4%) identities to other type 2 strains and only 44.9% and 29.7% nt and aa identities with type 1 strains LV.
ORFs 2 to 7 encoded the structural proteins of PRRSV. Sequence alignment indicated that GDsg shared 83.7% to 97.9% and 82.6% to 98.4% nt and aa identities, respectively, with other type 2 strains and only 62.9% to 71.6% nt and 54.3% to 80.5% aa identities with the type 1 strain LV.
To elucidate further the genetic relationship between GDsg and other reference PRRSV strains, phylogenetic trees based on the whole genomic sequence, the NSP2 nt sequence, and the ORF5 nt sequence were constructed by using a neighbor-joining method. As shown panel A in Fig. 1, the whole genomic sequences of the GDsg and reference strains was divided into the type 1 (European type: EU type) and type 2 (North American type: NA type) genotypes; moreover, the NA type was further divided into five subgenotypes. The NADC30 and the recently isolated NADC30-like strains in China belonged to subgenotype I, while the earlier isolated strains including American strain VR2332, Chinese strain BJ-4, Korea strain PL97-1, and Japan strain Jam2 were clustered into subgenotype II. GDsg belonged to subgenotype III along with GM2, QYYZ, and QY2010. The other representative China strain CH-1a and its cell-attenuated live virus vaccine strains CH-1R, HB-1, sh2002, and SHB, which are low pathogenic PRRSV strains, belonged to subgenotype IV. The JXA1 strain and its cell-attenuated live vaccine strain JXA1-P80, which are HP-PRRSV strains, belonged to subgenotype V. NSP2 and GP5 were two PRRSV proteins exhibiting the most variation, and those gene sequences had often been used as a target for analyzing genetic mutations in PRRSV. GDsg and the reference PRRSV strains formed a similar cluster when the phylogenetic tree was constructed based on NSP2 gene sequences with GDsg NSP2 belonging to subgroup III along with GM2, QYYZ, and QY2010 (panel B in Fig. 1). However, the phylogenetic tree had different branches when it was constructed based on the ORF5 gene sequences. As shown in panel C in Fig. 1, the GDsg ORF5 formed cluster subgroup I with the GM2, QYYZ, and QY2010 strains, while the other strains were divided into four other subgroups: II, III, IV, and V.
To describe further the characteristics of GDsg, the NSP2 and GP5 sequences were aligned with those of the reference PRRSV strains. As shown panel A in Fig. 2, NSP2 of GDsg had a 19-nt deletion at position 1404–1422, an 18-nt deletion at position 1426–1443, and a 94-nt deletion at position 1446–1540 when compared with VR2332, CH-1a, JXA1, JXA1-P80, and HuN4, suggesting that the reference strains had less similarity with GDsg than that of GM2, QYYZ, and QY2010, which had close similarity with GDsg. However, GDsg had a deficiency that was similar to that of NADC30 and the NADC30-like strain CHsx1401; that is, a complete nt deletion at position 1404–1540. In addition to such deletions, there were extensive insertions in the NSP2 region of the GDsg genome. Compared with the NA-type strain VR2332, the NSP2 of GDsg had a 108-nt insertion at position 2438–2545, which was the same as those of GM2, QYYZ, QY2010, and SP. Compared to Chinese strains CH-1a, HuN4, JXA1, and JXA1-P80, GDsg had a 107-nt insertion at position 2442–2549. Compared with NADC30 and the NADC30-like strain CHsx1401, GDsg also had a substantial nt insertion at positions 2454–2495 and 2498–2536.
Residues R13 and R151 of GP5 are related to PRRSV virulence [30], and, as shown panel B in Fig. 2, R13 and R151 of GDsg were substituted with Q13 and K151, in contrast to the residues in VR2332, CH-1a, HuN4, JXA1, JXA1-P80, and SP. The primary neutralizing epitope at position 37–44 of GP5 is important for inducing immune responses, and GP5 of GDsg had H38Y and L39S mutations, which differs from the sequence in VR2332. There are four potential N-glycosylation sites in the extravirion sequence of GP5 [2]. Compared with VR2332, GP5 of GDsg had N30S and N33G mutations, the same as the sequences in GM2, QYYZ, and QY2010. In addition, GP5 of GDsg had S12L, D61N, and H103R mutations in the signal peptide, extravirion, and intravirion regions, respectively, differing from the sequences in the reference strains.
The results from application of the NCBI's Nucleotide Blast software showed that the GDsg genome had the highest similarity with QYYZ, whereas NSP1β, NSP9, and NSP10 had their highest similarities with JXA1-P80. To examine further the recombination status of GDsg, we used GDsg as a query sequence and QYYZ and JXA1-P80 as the parent viruses in SimPlot software. As shown panel A in Fig. 3, the results show that GDsg was a recombinant strain between QYYZ and the cell-attenuated live virus vaccine strain JXA1-P80. Five recombination breakpoints were found at nt 161, 3021, 7459, 10601, and 13101 of the GDsg genome, which were located in the 5′UTR, NSP2, NSP7, NSP10, and GP3 regions, respectively, thus separating the genome into five regions. Two narrow zones (the first and third regions, designated region A) of GDsg had high similarity with JXA1-P80 and low similarity with QYYZ, the others (the second, fourth, and fifth regions, designated region B) had high similarity with QYYZ and low similarity with JXA1-P80. Phylogenetic analysis also indicated that region A was closely related to JXA1-P80 (panel B in Fig. 3) and that region B was closely related to QYYZ (panel C in Fig. 3).
The first PRRSV strain (VR-2332) was isolated in 1992 in North America and the first Chinese strain (CH-1a) was reported in 1996, and, since those isolations, PRRS has become one of the most troublesome swine diseases in the world and has led to huge economic losses [25]. Because of the immunosuppression caused by PRRSV and the host's immune system-induced selection pressure, it is difficult for the host to fully clear the virus. Under such conditions, the viral genome may mutate constantly in order to resist the host's selection pressure and more and more new mutated strains may emerge [163235]. In the present study, we isolated a new PRRSV strain, GDsg, and its genome's unique characteristics were analyzed.
Recent research involving phylogenetic analysis of PRRSV in China has shown that the isolated PRRSV strains commonly form into three clusters: subgroup I represented by VR-2332, subgroup II represented by CH-1a, and subgroup III represented by JAX1 [3137]. In our investigation, phylogenetic analysis indicated that the type 2 PRRSV isolates can be divided in five subgenotypes (I, II, III, IV, and V) and the representatives of these subgenotypes are NADC30, VR-2332, GM2, CH-1a, and JXA1, respectively. We observed that GDsg belonged within subgenotype III along with GM2, QYYZ, and QY2010, which were all isolated in South China in recent years. QY2010 is a HP-PRRSV that was isolated in 2010, while GM2 is a recombinant strain between vaccine strain MLV RespPRRS/Repro and a Chinese field strain QYYZ, which was isolated in 2012 [829]. Since 2006, a HP-PRRSV with a 30 aa deletion in the NSP2 region has been reported widely in China [16]. Since 2015, more and more NADC30-like strains have been isolated in China [1235]. In our current research, we have isolated new PRRSV strains with a high identity with GM2. Taken together, these results indicate that PRRSV has been undergoing continuous and extensive evolution. As a result, two new and different subgenotypes (I and III) might now be present in China.
The NSP2 gene is the most variable gene in the PRRSV genome and often exhibits deletion, insertion, and/or mutation [11]. In addition, the GP5 gene also has high genetic diversity [22]. Thus, both the NSP2 and GP5 genes can be used when examining PRRSV evolution. Phylogenetic analysis based on both the NSP2 and GP5 genes showed that GDsg formed a cluster with recombinant strain GM2, wild strain QYYZ, and QY2010, further supporting the observation that the GDsg genome belonged to a new cluster and might be a new recombined PRRSV strain.
In recent years, other atypical PRRSV strains have been reported, with novel nt deletions and insertions in NSP2 [1437]. In this study, sequence alignment indicated that NSP2 of GDsg had a 108-nt insertion at position 2438–2545, which was the same as those in GM2, QYYZ, and QY2010. However, in contrast to GM2, QYYZ, and QY2010, NSP2 of GDsg had a 19-nt deletion at position 1404–1422, an 18-nt deletion at position 1426–1443, and a 94-nt deletion at position 1446–1540. In addition, strains VR2332, CH-1a, JXA1, JXA1-P80 and HuN4 do not exhibit those deletions. The GP5 gene alignment in GDsg was different from the sequences in reference strains, with GP5 of GDsg having S12L, D61N, and H103R mutations in signal peptide, extravirion, and intravirion regions, respectively. These results indicate that GDsg had undergone large-scale mutation to become a novel PRRSV strain, and, even though GDsg has a high homology with GM2, QYYZ, and QY2010, those mutations might be the reason for its stronger virulence. More research is needed to confirm this possibility.
Abundant genetic mutation in a virus may increase its virulence and resistance to immune system-induced selection pressure. Previous reports have indicated that random mutation and intergenic recombination were two factors affecting PRRSV evolution [613]. Such recombination could result in large-scale mutation, resulting in new strains. A previous study showed that two different strains could recombine in MA-104 cells with new viral particles emerging [32]. In vivo study has indicated the presence of intragenic and intergenic recombinations in pigs infected with two different virulent PRRSV [18]. Shi et al. [26] reported that a recombination was related to the outbreak of HP-PRRSV in China. Since that outbreak, many actions have been taken to control the disease, and the use of attenuated live virus vaccines was the first choice; however, due to the extensive use of attenuated live virus vaccines and the presence of constant immune system-induced selection pressure, the probability of PRRSV recombination has increased [34]. Our results indicate that GDsg is an intragenic recombinant between wild strain QYYZ and attenuated live virus vaccine strain JXA1-P80, which is widely used in swine herds. Recently, Wenhui et al. [29] reported that vaccine strain RespPRRS MLV was associated with the emergence of recombinant PRRSV strain GM2. Furthermore, animal experiment results have indicated that the pathogenicity of recombinant GM2 is higher than that of the wild counterpart QYYZ [20]. Recombination appears to be important in PRRSV evolution; previously, Li et al. [15] reported that Chinese field strain Em2007 could recombine with vaccine variant HB-1(sh) resulting in a virulence increase through intragenic recombination. Our results indicate that the recombinant part of the GDsg genome included NSP1α, NSP1β, NSP9, and NSP10. Both NSP1α and NSP1β can suppress interferon and tumor necrosis factor-α production, and they have an important role in immune responses [527]. NSP9 and NSP10 encode PRRSV polymerase and helicase, respectively, which are involved in virus replication and RNA synthesis [17]. It has also been shown that NSP9 and NSP10 contribute to pathogenicity and the increasing HP-PRRSV virulence [17]. Recently, genomic variability in HP-PRRSV has been frequently observed, and the continuing changes in the genetics and antigenicity of field isolates are increasing the level of difficulty for controlling and eradicating PRRS in China. Therefore, further research into the pathogenic mechanism of recombined GDsg will be of benefit to elucidating fully the evolutionary characteristics of PRRSV in China.
In conclusion, a new PRRSV virulent strain, GDsg, was isolated and its whole genome was sequenced and characterized. The results indicate that GDsg belongs to a new subgenotype of PRRSV and has extensive nt deletions and insertions. Moreover, GDsg is a natural recombinant strain between a field strain and a vaccine strain. Considering the recent increases in the isolation of recombinant PRRSV strains, our results imply that recombination might be the main reason for the emergence of new virulent strains of PRRSV, and, importantly, the live vaccine prevention strategy currently in use in China could enhance this type of virus evolution, consequently threatening the swine industry.
Figures and Tables
Fig. 1
Phylogenetic trees based on the complete genome, NSP2, and ORF5 of PRRSV. (A) Complete genome-based tree. (B) NSP2 nucleotide-based tree. (C) ORF5-based tree. The isolate identified in this study is indicated by a black dot. NSP, nonstructural protein; ORF, open reading frame; PRRSV, porcine reproductive and respiratory syndrome virus; NA type, North American type; EU type, European type.

Fig. 2
Alignment of the partial NSP2 nucleotide sequence and the ORF5 translated amino acid sequence of GDsg with representative PRRSV strains. (A) Alignment of the partial NSP2 nucleotide sequence. The deleted regions are indicated by a red square and the inserted regions are indicated by an orange square. (B) Alignment of the ORF5 translated amino acid sequence. The signal peptide, transmembrane regions 1, 2, and 3 (TM1, TM2, and TM3) are indicated by a red square. NSP, nonstructural protein; ORF, open reading frame; PRRSV, porcine reproductive and respiratory syndrome virus.
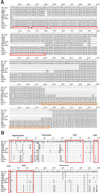
Fig. 3
Recombination analyses of the GDsg strain. (A) Similarity plot analysis using GDsg as the query sequence against those of JXA1-P80 (red) and QYYZ (green). Recombination breakpoints are shown as blue dotted lines. The minor parental regions are identified as region A, whereas that the major parental regions are identified as region B. (B) Phylogenies of parental regions A. (C) Phylogenies of parental regions B. The minor parental group (i.e., HP-PRRSV and related vaccine virus) is marked by asterisks; the major parental group (i.e., a new subgenotype in China) is marked by daggers. HP-PRRSV, highly pathogenic porcine reproductive and respiratory syndrome virus.

Acknowledgments
This work was supported by the National Key Technologies R&D Program (2015BAD12B02-5), the Guangzhou City Project (201508020062), and the Henan Science and Technology Project (172102110198), China.
References
1. Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997; 55:309–316.

2. Ansari IH, Kwon B, Osorio FA, Pattnaik AK. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J Virol. 2006; 80:3994–4004.

3. Bautista EM, Faaberg KS, Mickelson D, McGruder ED. Functional properties of the predicted helicase of porcine reproductive and respiratory syndrome virus. Virology. 2002; 298:258–270.

4. Bautista EM, Meulenberg JJ, Choi CS, Molitor TW. Structural polypeptides of the American (VR-2332) strain of porcine reproductive and respiratory syndrome virus. Arch Virol. 1996; 141:1357–1365.

5. Beura LK, Sarkar SN, Kwon B, Subramaniam S, Jones C, Pattnaik AK, Osorio FA. Porcine reproductive and respiratory syndrome virus nonstructural protein 1beta modulates host innate immune response by antagonizing IRF3 activation. J Virol. 2010; 84:1574–1584.

6. Chang CC, Yoon KJ, Zimmerman JJ, Harmon KM, Dixon PM, Dvorak CM, Murtaugh MP. Evolution of porcine reproductive and respiratory syndrome virus during sequential passages in pigs. J Virol. 2002; 76:4750–4763.

7. Conzelmann KK, Visser N, Van Woensel P, Thiel HJ. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993; 193:329–339.

8. Deng Y, Pan Y, Wang D, Zhou Q, Bi Y, Chen F, Song Y. Complete genome sequence of porcine reproductive and respiratory syndrome virus strain QY2010 reveals a novel subgroup emerging in China. J Virol. 2012; 86:7719–7720.

9. Feng Y, Zhao T, Nguyen T, Inui K, Ma Y, Nguyen TH, Nguyen VC, Liu D, Bui QA, To LT, Wang C, Tian K, Gao GF. Porcine respiratory and reproductive syndrome virus variants, Vietnam and China, 2007. Emerg Infect Dis. 2008; 14:1774–1776.

10. Forsberg R. Divergence time of porcine reproductive and respiratory syndrome virus subtypes. Mol Biol Evol. 2005; 22:2131–2134.

11. Han J, Wang Y, Faaberg KS. Complete genome analysis of RFLP 184 isolates of porcine reproductive and respiratory syndrome virus. Virus Res. 2006; 122:175–182.

12. Ji G, Li Y, Tan F, Zhuang J, Li X, Tian K. Complete genome sequence of an NADC30-like strain of porcine reproductive and respiratory syndrome virus in China. Genome Announc. 2016; 4:e00303–e00316.

13. Kapur V, Elam MR, Pawlovich TM, Murtaugh MP. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J Gen Virol. 1996; 77:1271–1276.

14. Li B, Fang L, Guo X, Gao J, Song T, Bi J, He K, Chen H, Xiao S. Epidemiology and evolutionary characteristics of the porcine reproductive and respiratory syndrome virus in China between 2006 and 2010. J Clin Microbiol. 2011; 49:3175–3183.

15. Li B, Fang L, Xu Z, Liu S, Gao J, Jiang Y, Chen H, Xiao S. Recombination in vaccine and circulating strains of porcine reproductive and respiratory syndrome viruses. Emerg Infect Dis. 2009; 15:2032–2035.

16. Li Y, Wang X, Bo K, Wang X, Tang B, Yang B, Jiang W, Jiang P. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the Mid-Eastern region of China. Vet J. 2007; 174:577–584.

17. Li Y, Zhou L, Zhang J, Ge X, Zhou R, Zheng H, Geng G, Guo X, Yang H. Nsp9 and Nsp10 contribute to the fatal virulence of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China. PLoS Pathog. 2014; 10:e1004216.

18. Liu D, Zhou R, Zhang J, Zhou L, Jiang Q, Guo X, Ge X, Yang H. Recombination analyses between two strains of porcine reproductive and respiratory syndrome virus in vivo. Virus Res. 2011; 155:473–486.

19. Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999; 73:152–160.

20. Lu WH, Tun HM, Sun BL, Mo J, Zhou QF, Deng YX, Xie QM, Bi YZ, Leung FC, Ma JY. Re-emerging of porcine respiratory and reproductive syndrome virus (lineage 3) and increased pathogenicity after genomic recombination with vaccine variant. Vet Microbiol. 2015; 175:332–340.

21. Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993; 192:62–72.

22. Murtaugh MP, Elam MR, Kakach LT. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol. 1995; 140:1451–1460.

23. Nelsen CJ, Murtaugh MP, Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J Virol. 1999; 73:270–280.

24. Nelson EA, Christopher-Hennings J, Drew T, Wensvoort G, Collins JE, Benfield DA. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J Clin Microbiol. 1993; 31:3184–3189.

25. Pejsak Z, Stadejek T, Markowska-Daniel I. Clinical signs and economic losses caused by porcine reproductive and respiratory syndrome virus in a large breeding farm. Vet Microbiol. 1997; 55:317–322.

26. Shi M, Holmes EC, Brar MS, Leung FC. Recombination is associated with an outbreak of novel highly pathogenic porcine reproductive and respiratory syndrome viruses in China. J Virol. 2013; 87:10904–10907.

27. Song C, Krell P, Yoo D. Nonstructural protein 1α subunit-based inhibition of NF-κB activation and suppression of interferon-β production by porcine reproductive and respiratory syndrome virus. Virology. 2010; 407:268–280.

28. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28:2731–2739.

29. Wenhui L, Zhongyan W, Guanqun Z, Zhili L, JingYun M, Qingmei X, Baoli S, Yingzuo B. Complete genome sequence of a novel variant porcine reproductive and respiratory syndrome virus (PRRSV) strain: evidence for recombination between vaccine and wild-type PRRSV strains. J Virol. 2012; 86:9543.

30. Wesley RD, Mengeling WL, Lager KM, Vorwald AC, Roof MB. Evidence for divergence of restriction fragment length polymorphism patterns following in vivo replication of porcine reproductive and respiratory syndrome virus. Am J Vet Res. 1999; 60:463–467.
31. Xie J, Zhu W, Chen Y, Wei C, Zhou P, Zhang M, Huang Z, Sun L, Su S, Zhang G. Molecular epidemiology of PRRSV in South China from 2007 to 2011 based on the genetic analysis of ORF5. Microb Pathog. 2013; 63:30–36.

32. Yuan S, Nelsen CJ, Murtaugh MP, Schmitt BJ, Faaberg KS. Recombination between North American strains of porcine reproductive and respiratory syndrome virus. Virus Res. 1999; 61:87–98.

33. Zhao K, Ye C, Chang XB, Jiang CG, Wang SJ, Cai XH, Tong GZ, Tian ZJ, Shi M, An TQ. Importation and recombination are responsible for the latest emergence of highly pathogenic porcine reproductive and respiratory syndrome virus in China. J Virol. 2015; 89:10712–10716.

34. Zhou L, Chen S, Zhang J, Zeng J, Guo X, Ge X, Zhang D, Yang H. Molecular variation analysis of porcine reproductive and respiratory syndrome virus in China. Virus Res. 2009; 145:97–105.

35. Zhou L, Wang Z, Ding Y, Ge X, Guo X, Yang H. NADC30-like strain of porcine reproductive and respiratory syndrome virus, China. Emerg Infect Dis. 2015; 21:2256–2257.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download