Abstract
We determined the threshold proportion of polymorphonuclear leukocytes (PMNs) for a diagnosis of cytological endometritis (CEM), the risk factors for this condition, and its impact on reproductive performance in dairy cows. Uterine cytology was performed on 407 Holstein cows 4 weeks postpartum to determine the proportions of endometrial cells and PMNs. A receiver operator characteristics curve was used to determine the threshold above which the PMN proportion affected the likelihood of cows conceiving by 200 days postpartum. The optimal threshold was ≥ 14% PMN (sensitivity, 31.3%; specificity, 81.7%; p < 0.05). The farm identity, retained placenta (odds ratio [OR] = 1.87), and septicemic metritis (OR = 3.07) were risk factors for CEM (p < 0.05). Cows with CEM were less likely to resume cyclicity (OR = 0.58) and to conceive by 200 days postpartum (hazard ratio = 0.58). Cows with CEM tended (p < 0.1) to be less likely to become pregnant after their first insemination (OR = 0.65) and to require a greater number of inseminations per conception (2.3 vs. 2.2). In conclusion, a PMN threshold of 14% defined the presence of CEM at 4 weeks postpartum. The farm, retained placenta, and septicemic metritis were risk factors for CEM, which reduces subsequent reproductive performance.
Endometritis is an important postpartum uterine disease that results in severe economic loss because of the expense of treatment, decreased production and reproductive performance, and increased culling of dairy cows [323]. It is defined as superficial inflammation of the endometrium in the absence of systemic signs [433]. Postpartum inflammatory conditions of the uterus commence with bacterial contamination of the uterine lumen and the influx of polymorphonuclear leukocytes (PMNs), which are attracted to the uterus because of the secretion of chemokines and have a key role in the uterine immune response [33].
Endometritis has been diagnosed by various methods, but no universally accepted definition of the disease being established [36]. Transrectal palpation and ultrasonography of the reproductive tract are commonly undertaken under field conditions [22135], while evaluation of vaginal mucus to define an inflammatory discharge through vaginoscopy or the Metricheck tool has also been used [2428]. A third method is the assessment of uterine cytology, which permits determination of the proportion of endometrial cells and PMNs, thereby defining cytological endometritis (CEM). Uterine cytology may be a more accurate method of diagnosis because it permits the differentiation of endometritis from other reproductive tract diseases, such as vaginitis or cervicitis, thus reducing the likelihood of making false positive diagnoses [1138]. Uterine cytology can be conducted by using a uterine cytobrush, lavage, or biopsy techniques [5101321]. The cytobrush method is more reliable than the lavage method, because the latter results in a higher degree of cellular distortion and is more time-consuming [20], while uterine biopsy is considered to be more invasive and expensive, as well as time-consuming [13].
In previous studies using uterine cytology, the optimal threshold level above which the proportion of PMNs significantly affected reproductive performance was evaluated in order to define CEM [221]. Kasimanickam et al. [21] set this threshold at > 18% PMNs at 20 to 33 days postpartum and at > 10% PMNs at 34 to 47 days postpartum, above which the percentage of cows becoming pregnant by 132 days postpartum was adversely affected. Another study [2] reported a threshold for PMNs of > 8% at 28 to 41 days postpartum, above which the chance of a cow becoming pregnant by 150 days postpartum was reduced. Thus, the PMN threshold used to define CEM could vary among herds, the timing of evaluation during the postpartum period, and the measure of reproductive performance employed [36].
The choices of an optimal postpartum evaluation period and an appropriate measure of reproductive performance are both likely to be important in defining the threshold proportion of PMNs necessary for the diagnosis of CEM. Uterine involution is complete by 25 to 30 days postpartum in normal dairy cows, but this process can be delayed under certain conditions, such as during uterine infection, which causes inflammation of the endometrium [253940]. Thus, the optimal time for evaluation of the endometrial inflammatory state is likely to be at 4 weeks postpartum, a time at which uterine involution should be complete. On the other hand, reproductive performance of dairy cows can be effectively evaluated by using the Cox proportional hazard model with the PHREG procedure provided in SAS software (ver. 9.4; SAS Institute, USA), which can be used to estimate the chance of a cow conceiving by a given time [22124]. Thus, we hypothesized that determination of the PMN percentage at 4 weeks postpartum, above which the subsequent reproductive performance of the cow is significantly impaired (defined by a reduced likelihood of conception by 200 days postpartum), might provide the most appropriate threshold to identify and thus control postpartum uterine disease.
In addition, determining the risk factors for CEM, including the biological condition of the cows and their environment (e.g., farm identity and calving season), might also provide useful additional information. Therefore, the objectives of this study were to determine the most appropriate PMN threshold for the diagnosis of CEM, the risk factors for this condition, and its impact on subsequent reproductive performance in dairy herds.
This study was conducted at five dairy farms, designated A–E, located in Chungcheong Province, Korea. Each herd consisted of between 95 and 250 cows. Cows were maintained in loose housing systems, fed total mixed rations, and milked twice daily. A total of 407 Holstein dairy cows (137 primiparous and 270 multiparous) were included in the study. All experiments were performed with the approval of the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea.
Uterine cytology samples were obtained at 4 weeks postpartum (28.3 ± 0.1 days) by using a cytobrush technique [21]. Briefly, a stainless-steel rod and cytobrush, which was guarded by a stainless-steel sheath, were introduced into the uterine body through the vagina and cervix. The stainless-steel sheath was retracted to expose the cytobrush, which was then rotated twice in a clockwise direction to obtain cells from the endometrium. After removing the steel device from the vagina, the cytobrush containing cellular material was rolled onto a glass slide and air dried. All slides were stained using a Diff-Quick stain kit (Sysmex, Japan), according to the manufacturer's instructions, and all slides were examined under a microscope (400× magnification) and by the same individual. The numbers of epithelial endometrial cells and PMNs were counted (up to 200 cells per slide) and the percentage of PMNs present was calculated (Fig. 1).
The definitions of the health disorders that were used in the present study were similar to those described previously [16262733]. Calving difficulty was ranked according to the degree of assistance required (1 = no assistance, 2 = minor assistance, 3 = some force required, 4 = significant force required, and 5 = cesarean section). Cows with a calving score > 2 were considered to have dystocia [27]. Retained placenta was defined as the retention of the fetal membranes for longer than 24 h [26]. Septicemic metritis was defined by the presence of fever (≥ 39.5℃) and a watery, fetid uterine discharge during the first 10 days postpartum [33]. Ketosis was diagnosed by the following clinical signs: anorexia, depression, and the odor of acetone on the breath [26]. Milk fever was diagnosed by the presence of weakness and recumbency after calving [26]. Abomasal displacement was diagnosed by the detection of a ‘ping’ sound during abdominal auscultation [16]. All cows received weekly reproductive health checks by the research team, including examination of ovarian structures, follicles, corpora lutea, and uterus by transrectal palpation and ultrasonography.
Resumption of cyclicity within 4 weeks of calving was evaluated by using ultrasonography and confirmed by detection of a corpus luteum during this period. The voluntary waiting period from calving to the first artificial insemination (AI) was 40 days. In addition to estrus detection, a herd reproductive management program was employed. Estrus synchronization was achieved through administration of prostaglandin F 2α (PGF2α) or Ovsynch [30]. Ovsynch was performed by administrating GnRH on Day 0, PGF2α on Day 7, and GnRH on Day 9. Cows that exhibited estrus naturally or after estrus synchronization using PGF2α were inseminated according to the am-pm rule. Cows treated with Ovsynch received timed AI. Pregnancy diagnosis was performed 32 to 40 days after AI and was based on transrectal palpation and ultrasonography. Reproductive performance data were collected for a minimum of 200 days postpartum or until pregnancy or culling.
In the first instance, a Cox proportional hazard model with PHREG procedure was used to evaluate whether the PMN proportion could be included as a factor affecting conception by 200 days postpartum. Then, a receiver operator characteristics (ROC) curve was generated to assess the optimal threshold above which the PMN proportion affected conception rate. The PMN levels tested were 5%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%, 20%, and 25%.
Once the threshold for the PMN proportion was set and the case definition was finalized for CEM, the risk factors for CEM and its impact on subsequent reproductive performance (the probability of resumption of postpartum cyclicity within 4 weeks of calving, probability of conception following the first insemination, intervals between calving and first insemination or pregnancy, and number of AIs required per conception) were determined.
Results are expressed as mean ± SEM values. For statistical analyses, calving season was defined as spring (March to May), summer (June to August), autumn (September to November), or winter (December to February), while cow parity was defined as primiparous or multiparous. Statistical analyses were performed by using the SAS software.
Cox's proportional hazard model with the PHREG procedure was used to analyze the proportion of cows being inseminated for the first time by 150 days and the proportion of cows conceiving by 200 days postpartum. This procedure provided estimates of the chance of a cow being inseminated or conceiving by a given time. The time variables used in this model were the interval in days between calving and first insemination, and the interval in days between calving and pregnancy. Cows that were sold, died, had not been inseminated by 150 days postpartum, or had not conceived by 200 days postpartum were excluded from the analyses. Cox models included farm identity (A–E), calving season, cow parity, PMN proportion (0%–92%), and the presence of CEM as variables. The proportional hazard rate was determined based on interactions between explanatory variables and time and by evaluating Kaplan-Meier curves. The median and mean days to first insemination or conception were determined by undertaking survival analysis using the Kaplan-Meier model and the LIFETEST procedure within SAS software. A survival plot was generated by using the survival option within MedCalc software (ver. 11.1; MedCalc Software, Belgium).
An ROC curve was constructed and the area under the curve determined to find the threshold that yielded the optimal combination of sensitivity and specificity for the proportion of PMNs that significantly affected the chance of conception by 200 days postpartum. The ROC curve was generated using the ROC curve analysis component of MedCalc software.
The risk factors for CEM and the probabilities of resumption of postpartum cyclicity and of conception following the first insemination were analyzed by logistic regression using the LOGISTIC procedure of SAS software. The logistic regression model for the risk factors of CEM included farm identity, calving season, cow parity, dystocia, and the presence of retained placenta, septicemic metritis, or metabolic disorders. The logistic regression models for resumption of postpartum cyclicity and probability of conception following the first insemination included farm identity, calving season, cow parity, and the presence of septicemic metritis or CEM. Backward stepwise regression was used in all models, and elimination based on the Wald statistic criterion was performed when p > 0.11. Odds ratios (ORs) and 95% confidence intervals (CIs) were determined by logistic regression. Results are presented as percentages and ORs with their respective 95% CIs.
Analysis of the number of AIs performed per conception was carried out by using the general linear model procedure. This model included farm identity, calving season, cow parity, and the presence of septicemic metritis or CEM.
A p value ≤ 0.05 was considered statistically significant, and 0.05 < p < 0.1 was considered to indicate a tendency toward significance.
The average PMN proportion per slide determined from 407 cytology samples from five dairy farms was 16.5 ± 1.2. The 407 cytology samples were used to determine the risk factors for CEM, whereas 309 and 378 cytology samples were used for the ROC curve and reproductive performance analyses, respectively, because not all samples were accompanied by follow-up reproductive data.
Table 1 lists the factors affecting the chance of conception by 200 days postpartum, analyzed by applying the PHREG procedure. Farm identity (p < 0.0001) and PMN proportion (hazard ratio [HR] = 0.99, p < 0.05) significantly affected this outcome, while cow parity also tended to have an effect (p < 0.1). However, calving season had no effect (p > 0.1). The likelihood of conception by 200 days postpartum was higher (HR = 1.65, p < 0.05) on farm B and lower (HR = 0.34, p < 0.0001) on farm D than on farm A.
The CEM incidence, sensitivity, specificity, positive predictive value, negative predictive value, area under the curve, and significance level at various PMN thresholds are presented in Table 2. The optimal threshold, which was accompanied by the maximum sensitivity and specificity for the PMN proportion, above which the chance of conception by 200 days was significantly affected, was 14% (Fig. 2). This proportion was used to define CEM at 4 weeks postpartum. The area under the ROC curve was 0.565 (CI = 0.507–0.621). The sensitivity and specificity for the prediction of conception by 200 days postpartum using this threshold level were 31.3% and 81.7%, respectively (p < 0.05). The prevalence of CEM using a PMN ≥ 14% threshold at 4 weeks postpartum was 31.1% (96 out of 309 cows).
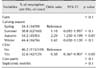
A logistic regression model revealed that the identity of the farm (p < 0.001) and the presence of retained placenta (p < 0.05) or septicemic metritis (p < 0.001) significantly affected the incidence of CEM (Table 3). However, calving season, cow parity, dystocia, and metabolic disorders did not have an effect (p > 0.1). The OR for the risk of CEM was lower on farms C (OR = 0.28, p < 0.01) and E (OR = 0.43, p < 0.05) than on farm A. Cows with a retained placenta (OR = 1.87) or septicemic metritis (OR = 3.07) had higher risks of CEM than cows without these problems.
Table 4 summarizes the ORs for variables included in the logistic regression model for the probability of resumption of postpartum cyclicity within 4 weeks of calving. CEM and calving season significantly affected this outcome (p < 0.05), while the farm, cow parity, and the presence of septicemic metritis had no effect (p > 0.1). Cows with CEM had a lower probability (OR = 0.58, p < 0.05) of resumption of cyclicity after calving than cows without CEM, whereas cows that calved during the autumn were more likely (OR = 2.29, p < 0.01) to resume postpartum cyclicity quickly than cows that calved during the spring. The mean interval between calving and first insemination did not differ between cows with and without CEM (100.7 ± 3.0 days and 91.2 ± 2.1 days, respectively; p > 0.1).
Cows with CEM were less likely to conceive after their first insemination (OR = 0.65, p < 0.1) than cows without CEM (Table 5), while cows with CEM tended (p < 0.1) to require more inseminations to conceive (2.3 ± 0.2) than cows without CEM (2.2 ± 0.1).
Using Cox's proportional hazard model with the PHREG procedure to analyze the chance of conception by 200 days postpartum revealed that farm identity (p < 0.0001) and CEM (HR = 0.74, p < 0.05) were significant factors, and cow parity also tended to have an effect (p < 0.1) (Table 6). However, calving season did not affect this outcome (p > 0.1). Cows with CEM took a median 47 days longer to conceive than cows without CEM, as shown by the survival curves generated using the survival option in MedCalc (Fig. 3). The chance of conception by 200 days postpartum was greater on farm B (OR = 1.63, p < 0.05) and lower on farm D (OR = 0.33, p < 0.0001) than on farm A.
This study determined the threshold for PMN proportion in uterine cytological results that could be used in the diagnosis of CEM, identified the risk factors for CEM, and determined its impact on subsequent reproductive performance in dairy cows. Our data show that the optimal threshold for PMN proportion of 14% was able to define CEM at 4 weeks postpartum, and that the farm, the presence of retained placenta, and septicemic metritis were risk factors for CEM. Furthermore, CEM impaired reproductive performance in dairy herds.
Establishing a numerical threshold for PMN percentage for use in defining CEM can be valuable for the monitoring of uterine health during the voluntary waiting period. Our results show that an increasing proportion of PMNs (0%–92%) can significantly affect the chance of conception by 200 days postpartum. Based on these results, we used ROC analysis to set an optimal PMN proportion threshold to define CEM at 4 weeks postpartum. The threshold was set at ≥ 14% PMN at 4 weeks postpartum in the present study. Similarly, Kasimanickam et al. [21] set the threshold level at > 18% PMN at 20 to 33 days postpartum, above which the PMN proportion affected the conception rate by 132 days postpartum. However, another study [2] reported that the threshold should be set at > 8% PMN at 28 to 41 days postpartum, above which the chance of conception by 150 days postpartum was affected. The reasons for these inter-study discrepancies are unclear. However, they might relate to herd identity, timing of evaluation (the period of time elapsed after calving), or the reproductive performance parameter used. Indeed, reproductive performance depends upon diverse factors including nutritional and healthy management [917], cow productivity, and/or the environment (climate) [815].
Our logistic regression model revealed that the identity of the farm and the presence of retained placenta or septicemic metritis were important risk factors for CEM. We found wide variation (15.7%–64.1%) in the incidence of CEM among farms, as reported (4.8%–52.6%) in a previous study [7]. The great variation in the incidence of CEM among the farms studied both in this study and previously [7] might be due to differences in production and management practices, and/or the environment (facilities, and/or barn conditions) [72229]. The OR (1.87) for the risk of a retained placenta in this study was lower than the OR (4.24) in a previous study [12], whereas the OR (3.07) for septicemic metritis in this study was higher than the OR (2.21) reported in another study [14]. Placental retention increases the incidence of CEM by establishing an environment that promotes infection because retained placental membranes provide an excellent medium for bacterial growth [632]. Metritis may also develop, which represents a chronic infection of the uterus that causes uterine inflammation [24].
In the present study, CEM adversely affected subsequent reproductive performance. A lower probability of cyclicity resuming within 4 weeks of calving might be the result of endocrine disturbances during the postpartum period. Bacterial endotoxin or lipopolysaccharide suppress the release of gonadotrophin-releasing hormone from the hypothalamus and luteinizing hormone from the pituitary, inhibiting ovulation of dominant follicles [1932]. Furthermore, cows with endometritis demonstrate slower growth of dominant follicles in the ovary and decreased estradiol secretion [3234] associated with down-regulation of aromatase transcripts in granulosa cells [18]. Our observations that cows calving during the autumn have a higher probability of resuming cyclicity rapidly than cows calving during the spring are consistent with the results of previous studies [3137]. It is likely that cows calving during the autumn would have been exposed to more favorable environmental conditions (e.g., temperature and humidity), which might have resulted in higher feed intake and better living conditions for cows. However, cows calving during the spring, especially late spring, might have been exposed to a less favorable environment (e.g., high temperatures and humidity), resulting in lower feed intake.
The decreases in reproductive performance (low probability of conception following the first insemination, more AIs required per conception, and lower chance of conception by 200 days postpartum) observed in cows with CEM in the present study are comparable to the results of several previous studies [121321]. The likelihood of AI being performed by 150 days postpartum was not affected by CEM in the present study, which is also consistent with a previous study [2]. However, other studies have reported that endometritis delayed the interval between calving and first service [1322].
In summary, the optimal PMN proportion threshold to be used in defining CEM at 4 weeks postpartum was 14%, above which there was a significant effect on subsequent reproductive performance based on the chance of conception by 200 days. This finding might provide useful guidance when monitoring postpartum uterine health and assessing subsequent interventions to improve reproductive performance. Improvements in management practices aimed at controlling postpartum diseases (such as retained placenta and septicemic metritis) are likely to be required to prevent CEM in dairy herds.
Figures and Tables
Fig. 1
Microscopic observation of the percentage of polymorphonuclear leukocytes (PMNs) among the total number of epithelial endometrial cells and PMNs obtained from uterine cytology samples (A: 0%, B: 15%, C: 25%, D: 50%). Large and small arrows indicate epithelial endometrial cells and PMNs, respectively. Diff-Quick stain, 400×. Scale bars = 20 µm (A–D).
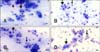
Fig. 2
Receiver operating characteristic curve representing the cytological endometritis threshold. The threshold was set at 14% polymorphonuclear leukocytes at 4 weeks postpartum (sensitivity, 31.3%; specificity, 81.7%; area under the curve, 0.565; p < 0.05).

Fig. 3
Survival curves for the interval between calving and conception in cows with and without cytological endometritis (CEM). The chance of conception by 200 days postpartum was lower (hazard ratio = 0.58; p = 0.0001) in cows with CEM than in cows without CEM. The median and mean days to conception were 191 and 160.6 ± 4.3 in cows with CEM, and 144 and 141.2 ± 3.3 in cows without CEM, respectively.

Table 1
Factors affecting the chance of conception by 200 days postpartum, analyzed by using the PHREG procedure in SAS software
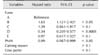
Table 2
CEM incidence, sensitivity, specificity, positive predictive value, negative predictive value, area under the curve, and significance level (p value) at various PMN thresholds

Table 3
Odds ratios for variables included in the logistic regression model of the risk factors for CEM
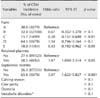
Table 4
Odds ratios of variables included in the logistic regression model for the probability of resumption of cyclicity within 4 weeks of calving
Acknowledgments
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (project No. PJ01081802)” Rural Development Administration, Republic of Korea.
References
1. Ahmadi MR, Kadivar A, Vatankhah M. Evaluation of polymorphonuclear (PMN) cells in cervical sample as a diagnostic technique for detection of subclinical endometritis in dairy cattle. Asian Pac J Reprod. 2016; 5:340–344.

2. Barlund CS, Carruthers TD, Waldner CL, Palmer CW. A comparison of diagnostic techniques for postpartum endometritis in dairy cattle. Theriogenology. 2008; 69:714–723.

3. Bartlett PC, Kirk JH, Wilke MA, Kaneene JB, Mather EC. Metritis complex in Michigan Holstein-Friesian cattle: incidence, descriptive epidemiology and estimated economic impact. Prev Vet Med. 1986; 4:235–248.

4. Bondurant RH. Inflammation in the bovine female reproductive tract. J Anim Sci. 1999; 77:Suppl 2. 101–110.

5. Bonnett BN, Martin SW, Gannon VP, Miller RB, Etherington WG. Endometrial biopsy in Holstein-Friesian dairy cows. III. Bacteriological analysis and correlations with histological findings. Can J Vet Res. 1991; 55:168–173.
6. Bruun J, Ersbøll AK, Alban L. Risk factors for metritis in Danish dairy cows. Prev Vet Med. 2002; 54:179–190.

7. Cheong SH, Nydam DV, Galvão KN, Crosier BM, Gilbert RO. Cow-level and herd-level risk factors for subclinical endometritis in lactating Holstein cows. J Dairy Sci. 2011; 94:762–770.

8. De Rensis F, Lopez-Gatius F, García-Ispierto I, Morini G, Scaramuzzi RJ. Causes of declining fertility in dairy cows during the warm season. Theriogenology. 2017; 91:145–153.

9. de Vries MJ, van der Beek S, Kaal-Lansbergen LM, Ouweltjes W, Wilmink JB. Modeling of energy balance in early lactation and the effect of energy deficits in early lactation on first detected estrus postpartum in dairy cows. J Dairy Sci. 1999; 82:1927–1934.

10. Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. Definitions and diagnosis of postpartum endometritis in dairy cows. J Dairy Sci. 2010; 93:5225–5233.

11. Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. Risk factors for postpartum uterine diseases in dairy cows. J Dairy Sci. 2010; 93:5764–5771.

12. Gautam G, Nakao T, Yusuf M, Koike K. Prevalence of endometritis during the postpartum period and its impact on subsequent reproductive performance in two Japanese dairy herds. Anim Reprod Sci. 2009; 116:175–187.

13. Gilbert RO, Shin ST, Guard CL, Erb HN, Frajblat M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology. 2005; 64:1879–1888.

14. Giuliodori MJ, Magnasco RP, Becu-Villalobos D, Lacau-Mengido IM, Risco CA, de la Sota RL. Clinical endometritis in an Argentinean herd of dairy cows: risk factors and reproductive efficiency. J Dairy Sci. 2013; 96:210–218.

15. Gröhn YT, Rajala-Schultz PJ. Epidemiology of reproductive performance in dairy cows. Anim Reprod Sci. 2000; 60-61:605–614.

16. Grosche A, Fürll M, Wittek T. Peritoneal fluid analysis in dairy cows with left displaced abomasum and abomasal volvulus. Vet Rec. 2012; 170:413.

17. Han YK, Kim IH. Risk factors for retained placenta and the effect of retained placenta on the occurrence of postpartum diseases and subsequent reproductive performance in dairy cows. J Vet Sci. 2005; 6:53–59.

18. Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, Sheldon IM. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction. 2007; 134:683–693.

19. Karsch FJ, Battaglia DF, Breen KM, Debus N, Harris TG. Mechanisms for ovarian cycle disruption by immune/inflammatory stress. Stress. 2002; 5:101–112.

20. Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, Walton JS, Johnson WH. A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can Vet J. 2005; 46:255–259.
21. Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, Walton JS, Johnson WH. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology. 2004; 62:9–23.

22. Kim IH, Kang HG. Risk factors for postpartum endometritis and the effect of endometritis on reproductive performance in dairy cows in Korea. J Reprod Dev. 2003; 49:485–491.

23. Kossaibati MA, Esslemont RJ. The costs of production diseases in dairy herds in England. Vet J. 1997; 154:41–51.

24. LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci. 2002; 85:2223–2236.

25. Leslie KE. The events of normal and abnormal postpartum reproductive endocrinology and uterine involution in dairy cows: a review. Can Vet J. 1983; 24:67–71.
26. Loeffler SH, de Vries MJ, Schukken YH. The effects of time of disease occurrence, milk yield, and body condition on fertility of dairy cows. J Dairy Sci. 1999; 82:2589–2604.

27. Lombard JE, Garry FB, Tomlinson SM, Garber LP. Impacts of dystocia on health and survival of dairy calves. J Dairy Sci. 2007; 90:1751–1760.

28. McDougall S, Macaulay R, Compton C. Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Anim Reprod Sci. 2007; 99:9–23.

29. Prunner I, Wagener K, Pothmann H, Ehling-Schulz M, Drillich M. Risk factors for uterine diseases on small- and medium-sized dairy farms determined by clinical, bacteriological, and cytological examinations. Theriogenology. 2014; 82:857–865.

30. Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. 1995; 44:915–923.

31. Santos JE, Rutigliano HM, Sá Filho MF. Risk factors for resumption of postpartum estrous cycles and embryonic survival in lactating dairy cows. Anim Reprod Sci. 2009; 110:207–221.

32. Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod. 2009; 81:1025–1032.

33. Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO. Defining postpartum uterine disease in cattle. Theriogenology. 2006; 65:1516–1530.

34. Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction. 2002; 123:837–845.

35. Studer E, Morrow DA. Postpartum evaluation of bovine reproductive potential: comparison of findings from genital tract examination per rectum, uterine culture, and endometrial biopsy. J Am Vet Med Assoc. 1978; 172:489–494.
36. Wagener K, Gabler C, Drillich M. A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology. 2017; 94:21–30.

37. Walsh RB, Kelton DF, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. Prevalence and risk factors for postpartum anovulatory condition in dairy cows. J Dairy Sci. 2007; 90:315–324.

38. Westermann S, Drillich M, Kaufmann TB, Madoz LV, Heuwieser W. A clinical approach to determine false positive findings of clinical endometritis by vaginoscopy by the use of uterine bacteriology and cytology in dairy cows. Theriogenology. 2010; 74:1248–1255.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download