Abstract
Enterotoxigenic Escherichia coli (ETEC) causes diarrhea in pigs, referred to as colibacillosis. The aim of this study was to optimize multiplex polymerase chain reaction (PCR) and immunohistochemistry (IHC) analyses of paraffin-embedded material to detect pathogenic E. coli strains causing colibacillosis in pigs. Multiplex PCR was optimized for fimbriae (F18, F4, F6, F5, and F41) and toxins (types A and B heat-stable toxins [STaP and STb], heat-labile toxin [LT], and type 2 Shiga toxin [STx2e]), and IHC was optimized for an anti-E. coli polyclonal antibody. Samples (132) from pigs received between 2006 and 2014 with clinical and histopathological diagnoses of colibacillosis were analyzed. E. coli was detected by IHC in 78.7%, and at least one virulence factor gene was detected in 71.2%. Pathogenic strains of ETEC with at least one fimbria and one toxin were detected in 40% of the samples in multiplex PCR. The most frequent virulence types were F18-STaP (7.5%), F18-STaP-STb (5.7%), and F4-STaP (3.8%). A statistically significant association was noted between virulence factors F4, F18, STaP, and STb and positive immunostaining results. Colibacillosis diagnosis through multiplex PCR and IHC of paraffin-embedded tissues is a practical approach, as samples can be fixed and stored for long periods before analysis.
Enterotoxigenic Escherichia coli (ETEC) causes diarrhea in neonatal and post-weaning pigs [5], and such infection (colibacillosis) presents as secretory diarrhea due to the action of enterotoxins that adhere to the intestinal mucosa, modifying water exchange and electrolyte flow in the small intestine [516]. The pathogenic potential of the ETEC agent is intimately related to its capacity to express virulence factors: fimbriae that allow intestinal colonization and enterotoxins that induce secretory diarrhea [913]. Overall, E. coli has been associated with significant economic losses in pigs due to morbidity, mortality, decreased weight gain, and increased treatment and vaccination costs [5].
A diagnosis of colibacillosis is obtained through clinical signs, epidemiological and pathological features, and a hallmark of the condition is the microscopic presence of coccobacilli adhered to the enterocyte surface [57]. E. coli identification can be performed by isolation from rectal swabs or intestine samples as well as through subsequent determination of virulence factors such as fimbrial adhesins and toxins. Previous studies have employed multiple primer pairs in a single polymerase chain reaction (multiplex PCR) to detect ETEC virulence factors from fresh tissues or bacterial isolates [31517].
The viability of bacterial cultures of E. coli is directly related to the time between the collection and processing of the samples; therefore, culture-based examinations should be performed rapidly to obtain better results [5]. In addition, paraffin-embedded tissues can serve as a source of material for use in PCR-based organism identification assays [11]. Nevertheless, the PCR-based approach presents some challenges, such as difficulties in extracting nucleic acids from fixed and paraffin-embedded materials due to the generation of cross-linkages in DNA and RNA; although these can be minimized through the employment of a carefully chosen extraction methodology [8]. However, detection of pathogenic strains of E. coli causing colibacillosis in pigs by using paraffin-embedded material has not yet been reported. Therefore, the aim of this study was to optimize multiplex PCR and immunohistochemistry (IHC) analyses of paraffin-embedded material to detect pathogenic strains of E. coli causing colibacillosis in pigs.
A retrospective study was performed through selection of paraffin-embedded samples of tissues from piglets that presented with diarrhea caused by E. coli with histopathological confirmation. Samples were obtained from swine in the southern region of Brazil. A total of 6,055 pigs were necropsied between 2006 and 2014 in our laboratory, of which 291 pigs (4.8%) were pigs at farrowing or weaning that had been diagnosed with diarrhea (multiple etiologies). Among these, 132 cases (45.4%) had a histopathological colibacillosis diagnosis obtained via hematoxylin and eosin staining and based on bacterial adherence to enterocytes. These cases included 35.6% (47/132) pigs at farrowing (0–21 days old), 53.0% (70/132) pigs at weaning (22–60 days old), and 11.4% (15/132) pigs of unknown age. In all 132 cases, paraffin-embedded small intestine fragments were still available, and, thus, were selected for inclusion in this study.
Paraffin-embedded tissues from piglets were restored from the necropsy database, and five dry 20-µm-thick sections were used for DNA extraction. A commercial kit for paraffin-embedded material (QiAamp DNA FFPE tissue kit; Qiagen, Germany) was used according to the manufacturer's instructions. Samples were pretreated for paraffin removal with a commercial deparaffinization solution (QiAamp; Qiagen). As positive controls for fimbriae (F18, F4, F6, F5, and F41) and toxins (types A and B heat-stable toxins [STaP and STb], heat-labile toxin [LT], and type 2 Shiga toxin [STx2e]), four E. coli reference samples in culture were kindly provided by the Departamento de Clínica e Cirurgia Veterinária da Universidade Federal de Minas Gerais, Brazil. These samples were identified as 2568 (STb, STaP, F18, and STx2e), 2569 (STb, LT, and F4 [K88]), 2570 (F6 [987P] and STaP), and 2571 (STaP, F5 [K99], and F41). In order to obtain isolated colonies, the reference samples were subcultured onto trypticase soy agar plates (Kasvi Brasil, Brazil), which were incubated for 24 h at 37℃. To confirm the identity of the colonies, new subculture onto MacConkey agar was performed, and, later, a final subculture onto brain heart infusion broth (Kasvi Brasil) for 18 h at 37℃ was performed. This culture was then centrifuged at 20,000 × g during five minutes for pellet formation. The bacterial pellet was fixed in 70% ethanol, and then embedded in a commercial specimen processing gel (Histogel; Thermo Fisher Scientific, USA) and routinely prepared for histopathology followed by paraffin embedding. From the positive control samples embedded in paraffin, five dry 20-µm-thick sections were obtained and used for DNA extraction.
The primers employed for detection of the target genes (F18, F4, F6, F5, F41, STaP, STb, LT, and STx2e) of this study were based on sequences described in previous studies of fresh E. coli strains [1415]; however, primer concentration and the remaining parameters of the multiplex PCR were distinct for the paraffin-embedded tissues. Primers (Applied Biosystems, USA) were tested at 10, 15, and 20 pmol. Annealing temperatures were tested with a gradient that varied from 53℃ to 58℃. Sample concentration varied from 1 to 3 µL. Three multiplex PCR systems (systems 1, 2, and 3; each detecting three genes) were developed for simultaneous identification of nine ETEC virulence factors. The optimized PCR protocol employed the following parameters: 200 nM of dNTPs, 15 pmol of each primer, 1 mM of MgCl2, 1× Taq DNA polymerase buffer 10, 1 unit of Taq DNA polymerase (Invitrogen Brasil, Brazil), and 2 µL of total DNA. The PCR was performed as follows: initial denaturation at 95℃ for 5 min, annealing at 55℃, 45 cycles at 95℃ for 30 sec and 72℃ for 45 sec, and a final extension at 72℃ for 5 min. PCR products were separated by performing agarose gel electrophoresis (2%) and DNA was viewed under ultraviolet light.
Primers for bacterial 16S ribosomal DNA (forward 5′-AGAGTTTGATCCTGGCTCAG-3′ and reverse 5′-GCGGCTGCTGGCACG-3′) were employed for the four E. coli reference strains embedded in commercial specimen processing gel to validate the DNA extraction from paraffin-embedded tissues.
To validate the specificity of the PCR products, a single PCR with specific primers targeting each fimbriae and toxin gene was tested. These PCR products were then purified with a PureLink PCR purification kit (Invitrogen, USA) and quantified with a Qubit fluorometric DNA quantification method (Invitrogen) following manufacturer's instructions in both. PCR products were then sequenced using a BigDye Terminator Cycle sequencing kit in an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems). Each sample was sequenced with forward and reverse primers, and the chromatogram results were analyzed by using Staden package software (ver. 1.7.0) [24].
Optimization of IHC was performed in 15 slides that contained two small intestine sections that were positive for E. coli enteritis (confirmed through bacterial isolation), and in 15 slides that contained cultivated reference bacterial samples embedded in paraffin with a commercial specimen processing gel. Tissue sections incubated with phosphate-buffered saline (PBS) (Sigma Chemical, USA) instead of the primary antibody served as negative controls. The samples were tested five times for each antigen retrieval protocol, which consisted of protease type XIV (15 min at room temperature) (Sigma Chemical), Tris-EDTA buffer pH 9.0 (digital pressure cooker for 20 min in a 96℃ water bath) (Synth; Labsynth, Brazil), and citrate buffer pH 6.0 (digital pressure cooker for 40 min in a 96℃ water bath and in a microwave oven) (Synth). Samples were subjected to IHC analysis via the streptavidin-biotin-peroxidase method, and endogenous peroxidase was blocked with 10% hydrogen peroxide for 15 min. Non-specific reactions were blocked with 5% skim milk for 15 min. The primary polyclonal anti-E. coli antibody (Virostat, USA) was tested at 1:200 and 1:100 dilutions in PBS and incubated overnight in a moist chamber at room temperature. The amplification signal used a biotinylated secondary antibody followed by a labeled streptavidin-biotin-peroxidase complex (DakoCytomation, USA) for 20 min each at room temperature. The reaction was developed by using a 3-amino-9-ethylcarbazole (AEC) chromogenic substrate (DakoCytomation). The slides were counterstained with Mayer's hematoxylin, and the tissues were covered by using coverslips in aqueous medium.
Samples selected from the necropsy database were tested with the optimized IHC protocol developed in this study. Tissue immunostaining was evaluated and graded according to stain intensity as follows: (−) absence of immunostaining for E. coli or immunostaining only of bacteria located in the intestinal lumen; (+) mild focal or multifocal areas of staining (approximately 25% of the section); (++) moderate focal or multifocal areas of staining (26% to 75% of the section); and (+++) marked diffuse staining (greater than 80% of the section).
Analysis of the frequency of virulence factor genes observed in the samples was performed by using a commercial software package (IBM SPSS Statistics ver. 22.0; IBM, USA). A chi-squared test was employed for comparison of the results obtained using both methods (multiplex PCR vs. IHC).
The three multiplex PCR systems (1, 2, and 3) are summarized in Table 1. The optimized PCR obtained a final volume of 25 µL (parameters previously described in Materials and Methods). For optimization of each system, multiplex PCR of ten replicates was performed. Only the targeted sequences were detected in the relevant positive controls, and there were no non-specific amplifications.
PCR for the bacterial 16S ribosomal DNA obtained positive results in all cases, demonstrating that the DNA extraction by the assay was efficient. In addition, PCR multiplex assays demonstrated that amplification products contained the expected base pairs numbers, confirming the efficacy of the tested primers. A consensus sequence for each sample was obtained, and, after sequence refinement, sequences were submitted to BLAST (National Center for Biotechnology Information, USA). The sequences showed 98% to 100% identity with sequences belonging to virulence gene factors previously registered in GenBank.
Immunostaining was observed after five replicates in all protocols for the cultivated reference bacterial samples embedded in paraffin with a commercial specimen processing gel. However, immunostaining was uneven (but located mainly in the intestinal lumen) when protease type XIV (Sigma Chemical) and Tris-EDTA buffer pH 9.0 (Synth) were employed in two small intestine sections from pigs infected with E. coli. Marked immunostaining, with the antigen mainly located in the intestinal lumen and adhered to enterocytes, was obtained in the positive controls when the samples were heated twice in citrate buffer pH 6.0 in a microwave oven for 5 min at maximum power and with a 1:200 primary antibody dilution. No immunostaining was detected in the negative control samples.
Based on the multiplex PCR assays, 71.2% (94/132) of the samples were positive for at least one of the virulence factor genes tested. The frequency of genes for fimbriae or toxins detected individually or in association with other factors was as follows: F18 (31.1%), F4/K88 (27.3%), F6/987P (15.2%), F5/K99 (13.6%), and F41 (9.1%). The frequency of toxins was as follows: STaP (39.4%), STb (28.0%), LT (17.4%), and STx2e (2.3%). The most frequent virulence types were F18-STaP (7.5%), F18-STaP-STb (5.7%), and F4-STaP (3.8%). A negative multiplex PCR result was obtained in 28.8% of the samples.
Based on the IHC analysis, 78.8% (104/132) of the samples were positive for E. coli (Fig. 1). This varied from mild (+) in 17.3% (18/104), moderate (++) in 26.9% (28/104), to marked (+++) in 55.8% (58/104) of the samples. In total, 82.7% (86/104) of the samples were positive by both IHC and multiplex PCR, whereas the remaining (17.3%) were only positive by IHC. Among the negative IHC results (28/132), eight were positive for virulence factor genes by multiplex PCR.
Statistical analysis revealed a correlation between both variables (multiplex PCR and IHC), with a significant association between virulence factors F4, F18, STaP, and STb with positive immunostaining (Table 2). The frequencies of virulence factors genes according to the levels of IHC staining and the frequencies of virulence factors genes according to the age range are presented in Supplementary Tables 1 and 2.
In this study, colibacillosis caused 45.4% (132/291) of the diarrhea observed in farrowing and weaning pig samples obtained via necropsy; a similar percentage has been reported previously (40.2%) [2]. In the present study, 71.2% of the samples (94/132) were positive for at least one of the virulence factor genes that were tested by performing multiplex PCR. Although this value was less than the 94.8% and 92% reported by other authors using cultivated bacterial assessment [2125], the results obtained through the extraction from paraffin-embedded tissues were similar to the previously reported 77% based on isolated E. coli assessment [26], and is greater than the 29.2%, 42%, and 62.9% based on multiplex PCR [141723]. The frequencies of virulence genes observed in the present study were similar to those in previous reports in which the virulence types for F18 and F4 fimbriae and for STaP and STb toxins were most frequent in cultivated bacteria [614212325]. The occurrence of high frequencies of F18 and F4 fimbrial genes may be explained by the fact that 53% of the samples were from weaning pigs, which is similar to that in a previous study that evaluated post-weaning E. coli isolates via multiplex PCR [26]. E. coli isolates expressing the F18 fimbrial gene have a predilection for colonizing pigs that are several weeks old more frequently than pigs in the early neonatal period [17]. During the weaning phase, expression of F18 receptors in intestinal epithelial cells is increased and F4 receptors are still being expressed [516]. STaP and STb toxin genes were observed at a higher frequency, which is common in diarrheic episodes in farrowing and weaning pigs [10]. The frequency of the STx2e toxin gene (2.3%) was less than those for STaP and STb, which is similar to results in previous studies [26]. Nonetheless, STx2e is known to be associated with edema disease in pigs and not with colibacillosis [5].
Pathogenic strains of ETEC adhere to enterocytes and produce enterotoxins and therefore require the expression of at least one fimbria and one toxin [5]. The present study revealed that 40% of the analyzed samples presented pathogenic ETEC, similar to previous reports of 54.8% and 50.5% occurrence [1423]. However, expression of a toxin gene with no fimbrial gene expression was observed in 13.6% (18/132) of the samples in the present study. It is possible that other fimbriae that were not included in our analysis were present, such as AIDA-I (autotransporter adhesin involved in diffuse adherence), which has been found in a large number of E. coli isolates associated with cases of diarrhea in farrowing and weaning piglets [12]. A study from Canada revealed a correlation between AIDA-I and F18 fimbriae in 4.1% of fecal samples from piglets with diarrhea [19], and similar results were reported by another group [28]. In the present study, 28.8% of the samples were negative for the virulence factor genes analyzed. Nonetheless, the samples might have contained one or more genes for fimbriae or toxins that were not included in the analysis, such as AIDA-I and enteroaggregative E. coli heat-stable enterotoxin 1 (EAST1), which have been described in E. coli strains isolated from diarrheic and non-diarrheic pigs [12]. AIDA-I has been detected previously in 26% of E. coli isolates from 4- to 6-week-old pigs [28], while EAST1 toxin has been previously detected by multiplex PCR in almost 64% of E. coli isolates from pigs with post-weaning diarrhea [26], demonstrating that other virulence factors may be involved in ETEC diarrhea.
More than one fimbria was detected in 27.3% of the samples, which is greater than that observed in a previous study of E. coli samples isolated from weaned piglets (6.3%) [23]. However, 26.5% of the samples in the current study exhibited more than one toxin, which is similar to a 37% occurrence reported previously [23]. The predominant virulence types identified in the present study (F18-STaP [7.5%], F18-STaP-STb [5.7%], and F4-STaP [3.8%]) were similar to those observed in previous reports: F18-STaP (11.1%), F4-LT-STaP-STb (9.8%), and F4-STaP (7.3%) [23]. Therefore, these three types appear to be the most frequent virulence types among weaned piglets with clinical signs of diarrhea in Southern Brazil. The distribution and frequencies of ETEC serotypes and pathotypes can vary considerably from region to region [26]; however, the frequencies observed in the current study and in a previous study [23] conducted in Southern Brazil (including many of the same cities as those in the current study) were similar. In the present study, the correlation between piglet age and frequency of virulence factors could not be analyzed effectively because most piglets belonged were in the weaning age range (70/132), with piglets at farrowing and unknown ages forming minor groups (47/132 and 15/132, respectively). In general, the frequency of E. coli virulence factors varies among different studies and depends on the age of the piglets, the analytical technique employed, the geographical location, and the samples examined [2421].
Based on IHC results, coccobacilli had adhered to the enterocyte surfaces of the small intestines in 78.8% (104/132) of the samples analyzed. This observation indicates that E. coli was present in the majority of the samples with a histopathological diagnosis of colibacillosis. Thus, the results of the IHC analysis were consistent with the presumptive diagnosis (based on clinical signs and histopathological lesions). IHC is an important diagnostic tool that uses 10% formalin-fixed paraffin-embedded tissue samples. This type of sample processing facilitates the shipment of samples to diagnostic laboratories, which may be located far from the collection site. In addition, this sample preparation technique enables retrospective studies to be conducted [1].
Among the positive samples detected by IHC, 82.7% (86/104) were also positive for genes encoding virulence factors when examined by multiplex PCR. These virulence factors are considered enterotoxigenic, which confirms the initial histopathological diagnosis of colibacillosis. However, 17.3% (18/104) of the samples were only positive in the IHC results. Nucleic acid quality within fixed specimens is highly dependent on pre-fixation factors (type of tissue and autolysis), fixation-related factors (type of fixative, pH, temperature, and duration of fixation) and post-fixation factors (temperature and duration of storage) [8]. Multiplex PCR in the IHC-only positive cases could have failed due to a prolonged duration of fixation and the quality of the fixative employed, which is usually not buffered. Potentially, this discrepancy between IHC and multiplex PCR may have been due to the polyclonal antibody employed in this study as it reacts with multiple epitopes of the antigen, allowing the immunostaining of several E. coli pathotypes. Furthermore, the inconsistent cases may have had virulence factors that were not analyzed in our multiplex PCR, such as the fimbria AIDA-I and the toxin EAST1 [12]. Another possibility is the presence of mutation in the regions analyzed, which could have resulted in amplification failure and, thus, a negative result [2123]. Eight samples were negative by IHC but positive for virulence factors by multiplex PCR. This discrepancy may have been due to a prolonged sample fixation time, as conformational changes in macromolecules occur when exposed to formalin, which may prevent the antibody binding to the antigen epitope [22].
Statistical analysis revealed a positive association between multiplex PCR and IHC results with presence of the F4, F18, STaP, and STb virulence factors correlated with positive immunostaining. Therefore, we suggest that the genes encoding F18 and F4 fimbriae and STaP and STb toxins were present in the cases of clinical diarrhea in piglets with positive IHC results for E. coli. These four genes were the most frequently detected in the present study as well as in other studies [62123]. Detection of F4 and F18 adherence factors has been suggested as an alternative method for colibacillosis diagnosis [6]. Previous studies have demonstrated that only the F4 and F18 fimbriae have significant importance in the occurrence of post-weaning diarrhea of swine in Denmark, Germany, and Hungary [182027], and the present study detected these two fimbriae in 58.4% of the samples examined. If multiplex PCR for all nine virulence factors assessed in this study cannot be performed, we suggest that PCR amplification of the F4, F18, STaP, and STb genes should be conducted.
In the present study, we optimized a multiplex PCR technique and developed a DNA extraction method from paraffin-embedded tissues for identification of ETEC virulence factors, and obtained frequencies that were similar to those in previous studies of multiplex PCR of isolated bacteria [614151721232526]. The multiplex PCR/IHC used in the present study facilitates sample shipment and sample analysis can be rapidly performed. Thus, it is proposed that a multiplex PCR/IHC approach should be used as an alternative under conditions in which the shipment of refrigerated samples for colibacillosis analysis to diagnostic laboratories is difficult; such an alternative allows the shipment of small intestine sections fixed in 10% formalin. Subsequent agent detection may be performed through both IHC analysis and multiplex PCR using paraffin-embedded samples, thereby allowing the diagnosis of colibacillosis in piglets.
Figures and Tables
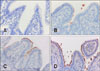 | Fig. 1Anti-Escherichia coli immunohistochemistry images of swine small intestine sections. (A) Negative control showing absence of immunostaining for E. coli. (B) Mild focal areas of immunostaining (up to 25% of the section). (C) Moderate multifocal areas of immunostaining (26%–75% of the section). (D) Marked diffuse immunostaining with multiple coccobacilli adhered to the enterocyte surface and covering the villi (greater than 80% of the section). Mayer's hematoxylin stain (A), Immunohistochemistry (AEC used) and Mayer's hematoxylin stain (B–D). 40× (A–D). |
Acknowledgments
We thank Professor Roberto Guedes for kindly providing the E. coli samples employed in the present study and Professor Caroline Argenta Pescador for the E. coli controls as well as her suggestion for the antibody employed for IHC. Conselho Nacional de Desenvolvimento Científico e Tecnológico, Fundação Estadual de Pesquisa Agropecuária, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior supported this study.
References
1. Arslan A, Saglam YS, Temur A. Detection of rabies viral antigens in non-autolysed and autolysed tissues by using an immunoperoxidase technique. Vet Rec. 2004; 155:550–552.

2. Boerlin P, Travis R, Gyles CL, Reid-Smith R, Janecko N, Lim H, Nicholson V, McEwen SA, Friendship R, Archambault M. Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Appl Environ Microbiol. 2005; 71:6753–6761.

3. Bosworth B, Casey T. Identification of toxin and pilus genes in porcine Escherichia coli using polymerase chain reaction (PCR) with multiple prime pairs. In : Abstracts of the 97th General Meeting of the American Society for Microbiology; 4-8 May, 1997; Miami Beach, USA.
4. Do TN, Cu PH, Nguyen HX, Au TX, Vu QN, Driesen SJ, Townsend KM, Chin JJ, Trott DJ. Pathotypes and serogroups of enterotoxigenic Escherichia coli isolated from pre-weaning pigs in north Vietnam. J Med Microbiol. 2006; 55:93–99.

5. Fairbrother JM, Gyles CL. Colibacillosis. In : Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. 10th ed. Ames: Blackwell Publishing;2012. p. 723–749.
6. Frydendahl K. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet Microbiol. 2002; 85:169–182.

7. Gelberg HB. Alimentary system and the peritoneum, omentum, mesentery and peritoneal cavity. In : Mcgavin MD, Zachary JF, editors. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis: Blackwell Publishing;2012. p. 374.
8. Gilbert MT, Haselkorn T, Bunce M, Sanchez JJ, Lucas SB, Jewell LD, Van Marck E, Worobey M. The isolation of nucleic acids from fixed, paraffin-embedded tissues-which methods are useful when? PLoS One. 2007; 2:e537.

9. Gyles CL, Prescott JF, Songer JG, Thoen CO. Pathogenesis of Bacterial Infections in Animals. 3rd ed. Ames: Blackwell Publishing;2004.
10. Hampson DJ. Post-weaning E. coli diarrhea in pigs. In : Gyles CL, editor. Esherichia coli in Domestic Animals and Humans. 1st ed. London: Cab International;1994. p. 171–192.
11. Hunt JL, Dacic S. Applications in anatomic pathology. In : Cagle PT, Allen TC, editors. Basic Concepts of Molecular Pathology. 1st ed. New York: Springer-Verlag;2009. p. 69–72.
12. Ikwap K, Larsson J, Jacobson M, Owiny DO, Nasinyama GW, Nabukenya I, Mattsson S, Aspan A, Erume J. Prevalence of adhesin and toxin genes in E. coli strains isolated from diarrheic and non-diarrheic pigs from smallholder herds in northern and eastern Uganda. BMC Microbiol. 2016; 16:178.

13. Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991; 4:80–128.

14. Macêdo NR, Menezes CPL, Lage AP, Ristow LE, Reis A, Guedes RMC. [Detection of pathogenic strains by multiplex PCR and antimicrobial sensitivity of Escherichia coli isolated from piglets]. Arq Bras Med Vet Zootec. 2007; 59:1117–1123. Portuguese.

15. Madoroba E, Van Driessche E, De Greve H, Mast J, Ncube I, Read J, Beeckmans S. Prevalence of enterotoxigenic Escherichia coli virulence genes from scouring piglets in Zimbabwe. Trop Anim Health Prod. 2009; 41:1539–1547.

16. Markey BK, Leonard F, Archambault M, Culinane A, Maguire D. Clinical Veterinary Microbiology. 2nd ed. St. Louis: Elsevier;2013.
17. Moon HW, Hoffman LJ, Cornick NA, Booher SL, Bosworth BT. Prevalences of some virulence genes among Escherichia coli isolates from swine presented to a diagnostic laboratory in Iowa. J Vet Diagn Invest. 1999; 11:557–560.

18. Nagy B, Nagy G, Meder M, Mocsári E. Enterotoxigenic Escherichia coli, rotavirus, porcine epidemic diarrhoea virus, adenovirus and calici-like virus in porcine postweaning diarrhoea in Hungary. Acta Vet Hung. 1996; 44:9–19.
19. Ngeleka M, Pritchard J, Appleyard G, Middleton DM, Fairbrother JM. Isolation and association of Escherichia coli AIDA-I/STb, rather than EAST1 pathotype, with diarrhea in piglets and antibiotic sensitivity of isolates. J Vet Diagn Invest. 2003; 15:242–252.

20. Ojeniyi B, Ahrens P, Meyling A. Detection of fimbrial and toxin genes in Escherichia coli and their prevalence in piglets with diarrhoea. The application of colony hybridization assay, polymerase chain reaction and phenotypic assays. Zentralbl Veterinarmed B. 1994; 41:49–59.

21. Post KW, Bosworth BT, Knoth JL. Frequency of virulence factors in Escherichia coli isolated from pigs with postweaning diarrhea and edema disease in North Carolina. Swine Health Prod. 2000; 8:119–120.
23. Sato JPH, Takeuti KL, Andrade MR, Koerich PKV, Tagliari V, Bernardi ML, Cardoso MRI, Barcellos DESN. Virulence profiles of enterotoxigenic Escherichia coli isolated from piglets with post-weaning diarrhea and classification according to fecal consistency. Pesq Vet Bras. 2016; 36:253–257.

25. Vidotto MC, de Lima NC, Fritzen JT, de Freitas JC, Venâncio MJ, Ono MA. Frequency of virulence genes in Escherichia coli strains isolated from piglets with diarrhea in the North Parana State, Brazil. Braz J Microbiol. 2009; 40:199–204.

26. Vu Khac H, Holoda E, Pilipcinec E, Blanco M, Blanco JE, Mora A, Dahbi G, López C, González EA, Blanco J. Serotypes, virulence genes, and PFGE profiles of Escherichia coli isolated from pigs with postweaning diarrhoea in Slovakia. BMC Vet Res. 2006; 2:10.
Supplementary Material
Supplementary data is available at http://www.vetsci.org only.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download