Abstract
It has been reported that Korean red ginseng (KRG), a valuable and important traditional medicine, has varied effects on the central nervous system, suggesting its activities are complicated. The paraventricular nucleus (PVN) neurons of the hypothalamus has a critical role in stress responses and hormone secretions. Although the action mechanisms of KRG on various cells and systems have been reported, the direct membrane effects of KRG on PVN neurons have not been fully described. In this study, the direct membrane effects of KRG on PVN neuronal activity were investigated by using a perforated patch-clamp in ICR mice. In gramicidin perforated patch-clamp mode, KRG extract (KRGE) induced repeatable depolarization followed by hyperpolarization of PVN neurons. The KRGE-induced responses were concentration-dependent and persisted in the presence of tetrodotoxin, a voltage sensitive Na+ channel blocker. The KRGE-induced responses were suppressed by 6-cyano-7-nitroquinoxaline-2,3-dione (10 µM), a non-N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, but not by picrotoxin, a type A gamma-aminobutyric acid receptor antagonist. The results indicate that KRG activates non-NMDA glutamate receptors of PVN neurons in mice, suggesting that KRG may be a candidate for use in regulation of stress responses by controlling autonomic nervous system and hormone secretion.
Ginseng (Panax ginseng Mayer) root has been used as a traditional medicine in many Asian countries and as an alternative medicine throughout the world [1]. Korean red ginseng (KRG) is a type of ginseng that, after cultivation for 4 to 6 years, goes through cleaning, steaming, and drying processes [44]. KRG has multiple underlying pharmacologic actions [1729]. It has been reported that KRG has several pharmacological functions including enhancing memory [16], anti-hypertensive [15], antitumor [45], anti-diabetic [41], erectile potentiation [6], and anti-stress [17]. Ginseng has a long history in Asia and is used for its wide spectrum of stamina enhancement and coping capacity functions associated with multiple stresses such as fear, anxiety, cold, heat, and pain as well as chemical or noxious stimuli [310121736]. Stress is a form of response to unexpected or perilous situations [30]. Recently, several studies have demonstrated that KRG has a beneficial role in the prevention of industrial (occupational) fatigue [17], inhibiting tumor metastasis [27], immunization against human immunodeficiency virus [37], and physicochemical stresses [18]. In addition, it has been shown that ocotillol, an extract from American ginseng, induces mitral cell activity through ionotropic glutamate receptors in vitro by using a whole-cell patch-clamp [42].
The paraventricular nucleus (PVN) neurons of the hypothalamus have a critical role in integrating autonomic and endocrine functions [39]. The PVN is a complex, heterogeneous structure adjacent to the third ventricle and is composed of magnocellular and parvocellular neurons, identified by their functions [38]. The magnocellular neurons release vasopressin or oxytocin into the posterior pituitary gland and into the bloodstream [731], and they show no low-threshold spikes after hyperpolarizing pulses larger than −100 mV [11]. The parvocellular neurons are composed of neurosecretory and preautonomic neurons and show discrete or bursting low-threshold spikes after hyperpolarizing pulses larger than −100 mV [11]. The parvocellular neurosecretory neurons synthesize regulatory hormones and their axons are involved in regulating secretion from the anterior pituitary gland and in collaborating with the hypothalamo-pituitary-adrenocortical (HPA) axis [3233].
Although many studies have reported action mechanisms of KRG on various cells and systems, the direct membrane effects of KRG on PVN neurons, which are involved in stress responses, have not been fully described. In the current study, the direct effects of a KRG extract (KRGE) on PVN neuronal activities were investigated by using a gramicidin perforated patch-clamp technique.
All experiments adhered to Chonbuk National University Animal Welfare and Ethics Committee's guidelines regarding the use and care of animals (protocol No. CBU-2014-0042). The ICR mice were housed under 12 h light/12 h dark cycles with free access to food and water.
Immature (8- to 18-day-old) male ICR mice were rapidly decapitated and the head placed in iced artificial cerebrospinal fluid (ACSF) of the following composition: NaCl 126 mM, KCl 2.5 mM, CaCl2 2.4 mM, MgCl2 1.2 mM, D-glucose 11 mM, NaH2PO4 1.4 mM, and NaHCO3 25 mM (pH = 7.4 and bubbled with O2 95% and CO2 5% between 10:00 and 12:00). The forebrain was blocked and glued to the chilled stage of a vibratome (Microme, Germany). Coronal slices (150–180 µm thick) including the PVN (bregma −0.58 to −1.22 mm) were cut and kept in the oxygenated ACSF for 1 h before use at room temperature.
For electrophysiological recording, a brain slice was transferred to a recording chamber and bathed with ACSF at a rate of 3 to 5 mL/min under continuous bubbling with O2 (95%) and CO2 (5%). The slices were fixed by a U-shaped platinum wire with single nylon strands and viewed through a microscope (BX51WI; Olympus, Japan). Glass pipettes of borosilicate capillary tubing (PG52151-4; WPI, USA) were made by using a puller (P-97; Sutter Instruments, USA). For perforated patch-clamp recording, a pipette solution containing (KCl 130 mM, NaCl 5 mM, CaCl2 0.4 mM, MgCl2 1 mM, HEPES 10 mM, and EGTA 1.1 mM [pH = 7.3 with KOH]) was passed through a 0.22 µm filter. Gramicidin (Sigma, USA) was dissolved in dimethyl sulfoxide to a concentration of 2 to 5 mg/mL and then diluted 1,000 times to a final concentration of 2 to 5 µg/mL. Access resistance was initially monitored and experiments were started when access resistance was stabilized at 50 to 90 MΩ. Stabilization typically took 15 to 20 min after gigaseal and, usually, resting was attained at under −45 mV. Membrane rupture was detected by a sudden overshoot of action potentials, after which the data were excluded. The membrane potential was sampled by using a Digidata 1322A digitizer (Axon Instruments, USA). Data acquisition and analysis were performed by using a Clampex clamp set (ver 9.0; Axon Instruments).
The KRGE was kindly provided by the Korea Ginseng Corporation (Korea). The obtained KRGE was reported to contain major ginsenosides including Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, and other minor ginsenosides (Korea Ginseng Corporation). Most test compounds were first dissolved in ACSF and then diluted by ACSF. CNQX (6-cyano-7-nitroquinoxaline-2, 3-dione) and tetrodotoxin (TTX) were purchased from Sigma and Tocris Bioscience (UK), respectively.
Data are expressed as mean ± SEM values. One-way ANOVA or a paired t-test was used for comparing more than three groups and two groups, respectively. When the test's p value was less than 0.05, it was considered to indicate a significant difference.
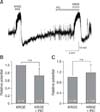
To determine whether KRGE can directly affect PVN neuronal activities, we applied KRGE in different concentrations. A low concentration of KRGE, less than 0.3 mg/mL, showed no notable changes in PVN neuronal activity. Thus, we applied KRGE ranging from 0.3 to 3.0 mg/mL. The KRGE-induced membrane potential changes gradually increased in proportion to the concentration (panel A in Fig. 1; 0.3–3.0 mg/mL). The mean membrane depolarizations by KRGE treatment concentration were 1.59 ± 0.78 mV (0.3 mg/mL, n = 7), 3.70 ± 1.33 mV (1.0 mg/mL, n = 7), and 9.45 ± 2.20 mV (3.0 mg/mL, n = 7), respectively (panel B in Fig. 1; p < 0.05, one-way ANOVA). The mean after-hyperpolarization values (i.e., depolarization followed by hyperpolarization) by KRGE concentration were −1.07 ± 0.66 mV (0.3 mg/mL), −1.91 ± 0.96 mV (1.0 mg/mL), and −4.53 ± 1.96 mV (3.0 mg/mL), respectively (panel C in Fig. 1; p > 0.05, one-way ANOVA). As the concentration of KRGE increased, after-hyperpolarization also tended to increase, but there was no a statistically significant difference.
To determine whether the KRGE-induced responses would be desensitized by repeated applications, we applied 3.0 mg/mL KRGE successively (panel A in Fig. 2). As shown in panel B in Fig. 2, the relative membrane depolarization value in the 2nd application of KRGE was 1.28 ± 0.19 times that in the 1st application (1st 8.25 ± 1.96 mV vs. 2nd 10.74 ± 2.70 mV, p > 0.05, n = 11, paired t-test). As shown in panel C in Fig. 2, the relative membrane after-hyperpolarization value in the 2nd application of KRGE was 1.34 ± 0.35 times that in the 1st application (1st −9.33 ± 2.28 mV vs. 2nd −9.87 ± 1.88 mV, p > 0.05, n = 7, paired t-test). Neither changes were significantly different.
To test whether the short-lived and reproducible membrane potential changes resulting from KRGE treatment are induced by direct action on PVN neurons, the effects of KRGE on the PVN neurons were investigated in the presence of TTX (0.5 µM), a voltage-gated sodium channel blocker. As shown in panel A in Fig. 3, KRGE-induced membrane potential changes persisted in the presence of TTX (n = 3). The membrane depolarization (13.4 ± 4.55 mV, n = 3) by KRGE in the presence of TTX was similar to the response by application of KRGE alone (11.5 ± 4.95 mV, n = 3). The after-hyperpolarization (−5.23 ± 4.08 mV, n = 3) by KRGE with TTX was similar to the response by application of KRGE alone (−6.38 ± 2.95 mV, n = 3). As shown in panels B and C in Fig. 3, the relative values of membrane depolarization and after-hyperpolarization with TTX were 1.31 ± 0.31 and 0.76 ± 0.38 times those of KRGE alone, respectively. Neither changes were significantly different (p > 0.05, n = 3, paired t-test). These data indicate that KRGE acts on the postsynaptic PVN neurons without action potential-mediated presynaptic events.
To determine which receptors are involved in the KRGE-mediated actions on PVN neurons, we applied KRGE in the presence of CNQX, a non-N-methyl-D-aspartate (NMDA) glutamate receptor antagonist. As shown in panel A in Fig. 4, the KRGE-induced depolarization (8.83 ± 1.45 mV, n = 5) and after-hyperpolarization (−3.9 ± 0.78 mV, n = 5) values decreased to 1.91 ± 1.17 mV and −0.16 ± 0.16 mV in the presence of CNQX, respectively. As shown in panels B and C in Fig. 4, the relative values of membrane depolarization and after-hyperpolarization in the presence of CNQX were 0.27 ± 0.17 and 0.06 ± 0.06 compared to those from the application of KRGE alone, respectively (p < 0.05, n = 5, paired t-test). Moreover, KRGE-mediated membrane depolarization was unaffected by the application of AP5 (20 µM), an NMDA receptor antagonist (data not shown). These results indicate that KRGE-mediated actions in PVN neurons are involved in the activation of non-NMDA glutamate receptors.
To determine whether the KRGE-induced membrane potential changes could be mediated by type A gamma-aminobutyric acid (GABAA) receptor activation in the PVN neurons, KRGE was applied in the presence of picrotoxin (PIC), a GABAA receptor antagonist. As shown in panel A in Fig. 5, the KRGE-induced responses (depolarization 9.13 ± 2.06 mV, n = 4; after-hyperpolarization −6.88 ± 3.88 mV, n = 3) were not affected by PIC (depolarization 7.79 ± 3.57 mV, n = 4; after-hyperpolarization −5.70 ± 1.85 mV, n = 3). As shown in panels B and C in Fig. 5, the relative values of membrane depolarization and after-hyperpolarization with PIC were 0.73 ± 0.30 (n = 4) and 1.17 ± 0.38 (n = 3) times those from application of KRGE alone, respectively (p > 0.05, paired t-test), indicating no significant effect by PIC application.
In the present study, we showed that KRG can activate non-NMDA glutamate receptors of the PVN neurons directly rather than via an action potential-mediated mechanism. Previous studies using whole-cell patch-clamp recordings showed that KRG activates GABAA and/or non-NMDA glutamate receptors on the preoptic gonadotropin-releasing hormone neurons [4] and on the medullary dorsal horn neurons [43] in mice. In the current study, the recordings were based on a gramicidin perforated patch-clamp, which can maintain the intracellular chloride intact and maintain cell virginity [19]. Based on the results of this study, the KRGE-induced membrane potential changes are accomplished through activation of non-NMDA receptors, not by the activation of GABAA receptors.
Currently, more than 30 ginsenosides, including Rb1–2, Rc, Rd, Rg1, Re, Rf, etc., have been isolated and reported to have pharmacological activity [10]. In addition, a number of studies have demonstrated that NMDA receptor-mediated Ca2+ and Na+ entries are suppressed by Rg3 application [5232425]. Some studies have shown that KRG-induced responses were mediated by the activation of the AMPA receptor, not by NMDA receptor activation [443]. Furthermore, it was shown that a low dose of decichine (a non-protein amino acid in roots of Panax notoginseng) contributes to reducing bleeding time by increasing the number of platelets via AMPA receptor activation [13]. The lack of AMPA receptors has contributed to enhancing the activity of the PVN neurons in hypertension rats [26]. Consequently, the effect of KRG through AMPA receptor demonstrates that KRG may be beneficial treatment for hypertension. Recently, it has been reported that gintonin is an important ginseng extract that can activate NMDA receptors to induce long-term potentiation via lysophosphatidic acid receptor (LPAR) mediation [34], and it can activate AMPA and NMDA receptors for synaptic enhancement by LPAR in the hippocampus [28]. In addition, gintonin can induce the release of epinephrine and norepinephrine through LPAR to increase the plasma glucose level, which may enhance physical stamina in mice [22]. The PVN neurons integrate and regulate the HPA axis to control catecholamine release [9]. Thus, among the many ingredients of KRG, gintonin may be a good candidate for further study into KRG's direct effects on PVN neuronal activities against stress and into determining the action mechanism involved.
In this study, KRGE induced membrane after-hyperpolarization in most cases in gramicidin perforated patch-clamp mode. Several studies have shown that after-hyperpolarization can be generated by activation of Ca2+-activated K+ channels [202135]. The AMPA receptor is one of non-NMDA glutamate receptors in the central nervous system, and it can be permeable to cations including Ca2+ [14]. It has also been reported that Ca2+-activated K+ channels are expressed in PVN neurons and have critical roles in control cardiac and renal functions [28]. In the neural network, AMPA is also involved in activation of Ca2+-activated K+ channels, which are associated with intracellular calcium kinetics [40]. The after-hyperpolarization in the PVN neurons can be explained by an intracellular calcium increase through KRGE-induced AMPA receptor activation followed by activation of Ca2+-activated K+ channels.
Since repeatable and concentration-dependent KRGE effects were not influenced by the presence of TTX, which blocks action potential-dependent transmission, we concluded that the KRGE-induced membrane potential changes were due to direct effects on the PVN neuron rather than via any action potential-mediated transmission. Direct effects of KRGE have also been reported on the gonadotropin-releasing hormone [4] and medullary dorsal horn neurons [43].
In conclusion, KRGE has excitatory effects on PVN neurons via non-NMDA glutamate receptors suggesting that KRG may be a potential target for applications regulating physiological and psychological stresses via its action on PVN neurons. However, further studies are needed to isolate and identify the compounds involved in the KRGE-mediated action mechanisms on PVN neurons.
Figures and Tables
Fig. 1
Concentration-dependent Korean red ginseng extract (KRGE) responses on paraventricular nucleus (PVN) neurons. (A) A representative trace from a PVN neuron showing concentration-dependent responses following application of KRGE (0.3, 1.0, and 3.0 mg/mL). Cumulative graphs show the mean membrane depolarization (B) and after-hyperpolarization (C) values after treatment by KRGE (0.3, 1.0, and 3.0 mg/mL). *p < 0.05.
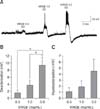
Fig. 2
Non-desensitizing responses by repeat applications of Korean red ginseng extract (KRGE) on paraventricular nucleus (PVN) neurons. (A) A representative trace from a PVN neuron showing depolarization and after-hyperpolarization after 3.0 mg/mL KRGE application. Mean relative membrane depolarization (B) and after-hyperpolarization (C) by repeat applications of KRGE (3.0 mg/mL). n.s., no significant difference.
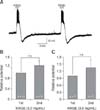
Fig. 3
Korean red ginseng extract (KRGE)-mediated membrane potential changes are induced by direct action on paraventricular nucleus (PVN) neurons. (A) A representative trace from a PVN neuron showing depolarization and after-hyperpolarization by application of 3.0 mg/mL KRGE alone and by KRGE (3.0 mg/mL) with tetrodotoxin (TTX) which blocks action potentials. Mean relative membrane depolarization (B) and after-hyperpolarization (C) by KRGE (3.0 mg/mL) alone and by KRGE (3.0 mg/mL) with TTX. n.s., no significant difference.
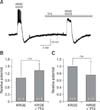
Fig. 4
Korean red ginseng extract (KRGE)-mediated membrane potential changes are mediated by activation of non-N-methyl-D-aspartate (NMDA) receptor glutamate receptors on paraventricular nucleus (PVN) neurons. (A) A representative trace from a PVN neuron showing depolarization and after-hyperpolarization following application of 3.0 mg/mL KRGE alone and KRGE (3.0 mg/mL) with CNQX (6-cyano-7-nitroquinoxaline-2, 3-dione), a non-NMDA glutamate receptor blocker. Mean relative membrane depolarization (B) and after-hyperpolarization (C) by KRGE (3.0 mg/mL) alone and by KRGE (3.0 mg/mL) with CNQX. *p < 0.05.
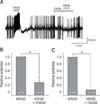
Fig. 5
Korean red ginseng extract (KRGE)-mediated membrane potential changes are not mediated by activation of type A gamma-aminobutyric acid (GABAA) receptor on paraventricular nucleus (PVN) neurons. (A) A representative trace from a PVN neuron showing depolarization and after-hyperpolarization following application of KRGE alone (3.0 mg/mL) and KRGE (3.0 mg/mL) with picrotoxin (PIC), a GABAA receptor antagonist. Mean relative membrane depolarization (B) and after-hyperpolarization (C) by KRGE (3.0 mg/mL) and KRGE (3.0 mg/mL) with PIC. n.s., no significant difference.
Acknowledgments
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A3B03932241).
References
1. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999; 58:1685–1693.
2. Brunton PJ, Sausbier M, Wietzorrek G, Sausbier U, Knaus HG, Russell JA, Ruth P, Shipston MJ. Hypothalamic-pituitary-adrenal axis hyporesponsiveness to restraint stress in mice deficient for large-conductance calcium- and voltage-activated potassium (BK) channels. Endocrinology. 2007; 148:5496–5506.

3. Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol. 1996; 23:728–732.

4. Cho DH, Bhattarai JP, Han SK. GABAA receptor- and non-NMDA glutamate receptor-mediated actions of Korean red ginseng extract on the gonadotropin releasing hormone neurons. J Ginseng Res. 2012; 36:47–54.

5. Choi SH, Lee JH, Pyo MK, Lee BH, Shin TJ, Hwang SH, Kim BR, Lee SM, Oh JW, Kim HC, Bae CS, Rhim H, Nah SY. Mutations Leu427, Asn428, and Leu431 residues within transmembrane domain-I-segment 6 attenuate ginsenoside-mediated L-type Ca2+ channel current inhibitions. Biol Pharm Bull. 2009; 32:1224–1230.

6. de Andrade E, de Mesquita AA, Claro Jde A, de Andrade PM, Ortiz V, Paranhos M, Srougi M. Study of the efficacy of Korean Red Ginseng in the treatment of erectile dysfunction. Asian J Androl. 2007; 9:241–244.

7. Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004; 25:132–149.

8. Feetham CH, Nunn N, Lewis R, Dart C, Barrett-Jolley R. TRPV4 and KCa ion channels functionally couple as osmosensors in the paraventricular nucleus. Br J Pharmacol. 2015; 172:1753–1768.

9. Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009; 517:156–165.

11. Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 2002; 87:2287–2296.

12. Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002; 25:861–866.

13. Huang LF, Shi HL, Gao B, Wu H, Yang L, Wu XJ, Wang ZT. Decichine enhances hemostasis of activated platelets via AMPA receptors. Thromb Res. 2014; 133:848–854.

14. Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M, Sasaki T, Ozawa S. Blockage of Ca2+-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002; 8:971–978.

15. Jeon BH, Kim CS, Kim HS, Park JB, Nam KY, Chang SJ. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000; 21:1095–1100.
16. Jin SH, Park JK, Nam KY, Park SN, Jung NP. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999; 66:123–129.

17. Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004; 95:158–162.

18. Kim HS, Jung MW, Jang CG, Park WK, Oh KW. Effects of ginseng total saponin on stress-induced analgesia. Korean J Ginseng Sci. 1993; 17:22–28.
19. Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995; 57:27–35.

20. Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986; 55:1268–1282.

21. Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987; 389:187–203.

22. Lee BH, Kim J, Lee RM, Choi SH, Kim HJ, Hwang SH, Lee MK, Bae CS, Kim HC, Rhim H, Lim K, Nah SY. Gintonin enhances performance of mice in rotarod test: involvement of lysophosphatidic acid receptors and catecholamine release. Neurosci Lett. 2016; 612:256–260.

23. Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Kim DH, Rhim H, Kim SS, Kim JI, Jang CG, Song JH, Nah SY. Characteristics of ginsenoside Rg3-mediated brain Na+ current inhibition. Mol Pharmacol. 2005; 68:1114–1126.

24. Lee JH, Jeong SM, Kim JH, Lee BH, Yoon IS, Lee JH, Choi SH, Lee SM, Park YS, Lee JH, Kim SS, Kim HC, Lee BY, Nah SY. Effects of ginsenosides and their metabolites on voltage-dependent Ca2+ channel subtypes. Mol Cells. 2006; 21:52–62.
25. Lee JH, Lee BH, Choi SH, Yoon IS, Shin TJ, Pyo MK, Lee SM, Kim HC, Nah SY. Involvement of batrachotoxin binding sites in ginsenoside-mediated voltage-gated Na+ channel regulation. Brain Res. 2008; 1203:61–67.

26. Li DP, Byan HS, Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci. 2012; 32:372–380.

27. Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995; 18:1197–1202.

28. Park H, Kim S, Rhee J, Kim HJ, Han JS, Nah SY, Chung C. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015; 113:1493–1500.

29. Park JD. Recent studies on the chemical constituents of Korean ginseng (Panax ginseng C. A. Meyer). Korean J Ginseng Sci. 1996; 20:389–396.
31. Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol. 1991; 36:131–169.

32. Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovács KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996; 107:201–222.
33. Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000; 122:61–78.

34. Shin TJ, Kim HJ, Kwon BJ, Choi SH, Kim HB, Hwang SH, Lee BH, Lee SM, Zukin RS, Park JH, Kim HC, Rhim H, Lee JH, Nah SY. Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-D-aspartic acid receptor activation: involvement of LPA receptors. Mol Cells. 2012; 34:563–572.

35. Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987; 385:733–759.

36. Sugaya A, Yuzurihara M, Tsuda T, Yasuda K, Kajiwara K, Sugaya E. Proliferative effect of ginseng saponin on neurite extension of primary cultured neurons of the rat cerebral cortex. J Ethnopharmacol. 1988; 22:173–181.

37. Sung H, Jung YS, Cho YK. Beneficial effects of a combination of Korean red ginseng and highly active antiretroviral therapy in human immunodeficiency virus type 1-infected patients. Clin Vaccine Immunol. 2009; 16:1127–1131.

38. Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983; 6:269–324.

39. Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980; 31:410–417.

40. Tegnér J, Lansner A, Grillner S. Activity dependent modulation of the burst rate by calcium-dependent potassium channels in lamprey. In : Bower JM, editor. Computational Neuroscience. Boston: Springer;1998. p. 549–554.

41. Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008; 18:46–56.

42. Wang ZJ, Sun L, Peng W, Ma S, Zhu C, Fu F, Heinbockel T. Ginseng derivative ocotillol enhances neuronal activity through increased glutamate release: a possible mechanism underlying increased spontaneous locomotor activity of mice. Neuroscience. 2011; 195:1–8.

43. Yin H, Park SA, Park SJ, Han SK. Korean red ginseng extract activates non-NMDA glutamate and GABAA receptors on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. J Ginseng Res. 2011; 35:219–225.

44. Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001; 16:Suppl. S3–S5.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download