Abstract
Tibial dyschondroplasia (TD) cases has not been reported in Tibetan chickens (TBCs), but it is commonly seen in commercial broilers characterized by lameness. The underlying mechanism remains unclear. Hypoxia-inducible factors (HIFs) are important regulators of cellular adaptation to hypoxic conditions. In this study, we investigated the role of HIF-1α, -2α, and -3α in hypoxia and thiram-induced TD and their effect on tibial growth plate development in Arbor Acres chickens (AACs) and TBCs. RNA and protein expression levels of HIF-1α, -2α, and -3α were determined by using quantitative reverse transcriptase polymerase chain reaction and western blotting analyses, respectively. Interestingly, the results showed that HIF-1α, -2α, and -3α expressions in the tibial growth plate of TBCs were upregulated by hypoxia and the change was more significant in TBCs than in AACs. However, these factors were downregulated in thiram-induced TD. To further clarify the effect of thiram on tibial growth plate in commercial broilers, AACs were observed to exhibit more pronounced changes in their growth plate that that in TBCs. Taken together, these results demonstrate that HIF-1α, -2α, and -3α may be important in tibial growth plate development and in the prevention of TD. The present study contributes novel insights on a therapeutic target for poultry TD.
Tibial dyschondroplasia (TD) is a particularly common leg problem worldwide with unknown natural etiology in commercial broilers and is characterized by abnormal proximal tibial bone formation and avascular cartilage [51618]. The avascular and noncalcified cartilage leads to an apparent locomotion problem, and there is a prevalence of approximately 30% (and rising) in broiler flocks [1518]. In general, precise prevalence estimates of TD are not readily accessible due to its mostly sub-clinical symptoms [5], but TD does result in leg weakness and motion reduction, which contribute to reduced production performance and compromised poultry welfare.
Tibetan chickens (TBCs) are a native poultry breed distributed on the Tibetan Plateau (2,600–4,500 m above sea level), where the environment is highly oxygen deficient (hypoxic) compared with that at sea level [1221]. We noticed that this unique breed has never been reported to exhibit leg disorders, especially TD, which may be related to the long-term growth of TBC populations in a high altitude hypoxic environment [9].
Hypoxia-inducible factors (HIFs) consist of two regulatory factors, HIF-α and HIF-β, which act as defense mechanisms against hypoxia [23]. As a transcription factor, HIF-1α can be activated in a hypoxic microenvironment and has been subdivided into three subunits: HIF-1α, HIF-2α, and HIF-3α [4]. HIF-α is a key regulator in the induction of genes that promote tumor angiogenesis and growth under normoxia and hypoxia [7], and they modulate chondrogenesis [8]. HIF-2α, generally recognized as endothelial PAS domain-containing protein 1 (EPAS-1), is associated with a reduction in cellular oxygen tension, is very similar to HIF-1α, and is expressed in chondrocytes [22025]. The biological role of HIF-3α is incompletely described; however, it is consistently transformed during chondrogenesis by controlling the stability of the chondrocyte phenotype [13]. Numerous studies have highlighted the upregulation of HIF expression under hypoxic conditions [132833], and HIFs are regarded as vital transcription factors because of their role in angiogenesis [37], cell differentiation [8], and cell apoptosis [129]. However, the HIF-1α, -2α, and -3α mechanisms in thiram-induced poultry TD remain unclear. Therefore, this study was designed to determine the molecular mechanisms of HIF-1α, -2α, and -3α in thiram-induced TD of TBCs and to investigate the absence of TD in TBCs reared in a high altitude hypoxic environment. As HIF-1α, -2α, and -3α may be important for development of the tibial growth plate and may act as a protective shelter against TD, the results of this study may provide new insights into a potential therapeutic agent for poultry TD.
This experiment was conducted after considering all national legislation regarding animal welfare and protection and followed the instructions and approval of the Institution Animal Care and Use Committee of Huazhong Agricultural University, Wuhan, China (approval permit No. 31272517). Before exsanguination and necropsy, injection of pentobarbital was used in conjunction with standard euthanasia protocols to minimize animal suffering and provide a humane death.
One-day-old healthy TBCs (n = 240) were purchased from a commercial hatchery at Lhasa, China and shipped to the laboratory of the Tibet Agricultural and Animal Husbandry College (approximately 3,000 m above sea level) on the same day. Simultaneously, one-day-old healthy Arbor Acres chickens (AACs, n = 240) were purchased from a private hatchery at Chengdu, China and transported to the same laboratory. The chicks were raised in two-layer metal cages (size, 80 × 60 × 50 cm3) for 14 days. Brooding temperature was maintained at 33℃ to 35℃ during the first week and steadily reduced to 29℃ at the end of second week. During the experiments, daily lighting was fixed with 23 h of light and 1 h of dark. Additionally, feed and water were provided ad libitum and the nutrient content of the feed (220 g/kg crude proteins and 12.6 MJ metabolizable energy/kg of diet) was as suggested by the National Research Council (1994).
All chicks were randomly assigned to two groups: a hypobaric normoxia group and a natural hypoxia group (approximately 21% oxygen content and natural oxygen content, respectively; n = 60 per group, 4 cages per treatment, and 15 chicks per cage). Oxygen content of the hypobaric normoxia group was maintained by using an oxygen generator (Yuwell, China). The oxygen content of the normoxia and hypoxia groups was monitored throughout the experiments by using a gas detector (CY-7B; Oxygen Analysis Instrument Factory, China).
TD was induced as described by Nabi et al. [14]. Both TBCs and AACs were divided into two separate groups: control group (normal diet) and thiram-induced TD group (normal diet plus 50 mg/kg of thiram [tetramethyl thiuram disulphide] during day 4 to day 7 of the trial). All chicks were reared at high altitude with normal oxygen content provided by an oxygen generator.
Two chicks were randomly chosen from each treatment cage (n = 8/treatment) at days 3, 7, 10, and 14 of the experiment. All group chickens were euthanized by cervical dislocation following pentobarbital injection. Subsequently, the tibia were retrieved and growth plates were detached from the articular cartilage of the tibia via surgical knife and instantly frozen in liquid nitrogen as previously described [31].
By using TRIzol reagent (Life Technologies, USA), total RNA was removed from each sample (approximately 100 mg) of primary tibial growth plate cartilage collected from each group. The cDNA was produced via EasyScript One-Step gDNA Removal and a cDNA Synthesis Kit (TransBionovo, China) as per the instructions of the manufacturers. Reverse transcription was performed by using reaction temperatures of 42℃ (15 min) and 85℃ (5 sec) in an applied thermocycler (Biosystems, USA). The cDNA was synthesized in a total volume of 25 µL of reaction mixture containing oligo(dt)18, 2 × ES reaction mix, gDNA remover, RT enzyme mix, and 2,000 ng of RNA. Finally, the target genes were identified via agarose gel electrophoresis.
A specific primer set was prepared based on Gallus gallus published sequences (Table 1), quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed with a TransStart Tip Green qPCR Kit (TransBionovo, China) in a Step One-Plus Real-Time PCR System (Applied Biosystems, USA). The procedure was as follows: 1 cycle of 30 sec at 94℃, and 40 amplification cycles of 5 sec at 94℃ and 30 sec at 60℃, and 72℃ for 30 sec. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control and the relative expression of each target gene was checked by assessing comparative threshold cycle (CT) values.
Tibia growth plates were homogenized in ice-cold buffer and incubated at 4℃ for 2 h. The samples were centrifuged at 2,000 × g for 10 min to collect the supernatant (total protein) and the sample concentrations were determined by using a BCA protein quantitative detection kit (Servicebio Technology, China), after which all samples were cryopreserved at −70℃ for subsequent use. Protein samples were separated by SDS-PAGE on 12% polyacrylamide gel until the dye band reached the end of the gel and were then transferred to polyvinylidene difluoride membranes, which were then incubated in 5% skimmed milk at room temperature for 1 h. The membranes were incubated overnight at 4℃ with rabbit monoclonal anti-HIF-1α primary antibodies (1:1,000 dilution, A11945; ABclonal Technology, China). The membranes were washed 3 times with PBS Tween 20 for 5 min each, then incubated with a secondary antibody (1:3,000 dilution; horseradish peroxidase labeled rabbit anti-goat secondary antibodies) for 1 h at room temperature. After washing, the bands were visualized and exposed by chemiluminescence and radiography, respectively. Band images were obtained by using an EPSON V300 imaging system (EPSON, China).
Comparisons of two groups were carried out by using Student's t tests. For TD morbidity, Chi-squared analysis was used for each group and performed via GraphPad Prism5 (GraphPad Software, USA). A p value < 0.05 level was used as indicative of statistical significance and group values are presented as means ± SD. All analyses were performed by using SPSS Statistics software (ver. 17.0; SPSS, USA).
To examine the transcriptional levels of HIF-1α, -2α, and -3α in tibial growth plates of TBCs, we performed qRT-PCR for HIF-1α, -2α, and -3α using tissues obtained from the early phase tibia (days 3, 7, 10, and 14, respectively) of TBCs. As showed in panels B to D in Fig. 1, there were significant increases in expression levels of HIF-1α on day 10 (p < 0.001), HIF-2α on days 3 (p < 0.001), 7 (p = 0.024), 10 (p = 0.012), and 14 (p = 0.043), and HIF-3α on days 7 (p = 0.041), 10 (p = 0.034), and 14 (p < 0.001) in the tibial growth plate of the hypoxia group as compared to expressions in the normoxia group. In addition, western blotting results revealed a clear increase in protein level of HIF-1α in TBCs under high altitude hypoxic conditions (panel A in Fig. 1).
To clarify the effect of hypoxia on TBCs and AACs, we used TBCs reared at high altitude for assessment of natural hypoxia effects and AACs for assessment of normoxia effects. Similar changes in HIF-1α, -2α, and -3α expression in the tibial growth plate are shown in panels B to D in Fig. 2. Compared to the AACs, the expression level of HIF-1α in TBCs showed significant increases on days 3, 7, 10, and 14 (p = 0.005, p = 0.001, p < 0.001, and p < 0.001, respectively); similarly, there were increases in HIF-2α on days 7, 10, and 14 (p = 0.017, p < 0.001, and p = 0.047, respectively) and in HIF-3α on days 3, 7, 10, and 14 (p = 0.021, p < 0.001, p = 0.005, and p < 0.001, respectively). Western blotting further confirmed HIF-1α expression changes, showing significant increases in TBCs compared to AACs (panel A in Fig. 2).
The morbidity rate of TD in TBCs was 61.7% (37/60) during days 4 to7 of treatment with thiram, and a significant difference (p = 0.002) was observed between AACs and TBCs (Table 2). In addition, we were surprised to observe that thiram not only induced TD in TBCs, but it also decreased the transcriptional levels of HIF-1α, -2α, and -3α in tibial growth plates. As shown in panels B to D in Fig. 3, there was significant variation between the control and thiram groups. In the control and thiram-treated TBC groups, the expression levels of HIF-1α (p = 0.009 and p = 0.036, respectively), HIF-2α (p = 0.002 and p = 0.001, respectively), and HIF-3α (p = 0.003 and p < 0.001, respectively) of the thiram group were significantly lower on days 7 and 10 than those in the control group. In addition, western blotting results showed an obvious decrease in the protein level of HIF-1α in the thiram-treated group (panel A in Fig. 3).
To clarify further the effect of thiram on commercial broilers, we used AACs in a thiram-induced TD experiment. The results demonstrated that the expression intensity of HIF-1α in the tibial growth plate was significantly decreased on days 7 and 10 (p < 0.001 and p = 0.022, respectively); however, HIF-1α expression was markedly elevated on day 14 (p < 0.001) (panel B in Fig. 4). Similarly, the expression level of HIF-2α was obviously reduced on days 7 and 10 (p = 0.002 and p = 0.001, respectively) as was HIF-3α on days 7, 10, and 14 (p = 0.007, p = 0.004, and p = 0.006, respectively) (panels C and D in Fig. 4). Similarly, western blotting revealed an obvious decrease in protein level of HIF-1α in thiram-treated AACs (panel A in Fig. 4). In addition, in a comparison of thiram-induced TD in TBCs and AACs, we observed that the suppressed HIF-1α, -2α, and -3α expression levels in TBCs exhibited better recovery on day 10 than that in AACs (Figs. 3 and 4).
TD is an abnormal skeletal disorder of broiler chickens that leads to abnormal formation of tibia along with the occurrence of non-vascularized and non-mineralized cartilage in tibial growth plates [101618]. TD is very challenging because of its unknown etiology. Prior to this study, we noticed that TD in TBCs had never been reported and speculated that that absence may be associated with long-term presence of TBCs in a high altitude hypoxic environment. The HIF pathway is the essential regulator of adaptive reactions to hypoxia and is a critical mediator of neoangiogenesis [112427]. Our qRT-PCR and western blotting analyses showed significantly elevated expression levels of HIF-1α, -2α, and -3α in the tibial growth plate of TBCs under natural hypoxia conditions compared to those in the normoxia group of TBCs. Upregulation of HIF-1α, -2α, and -3α may further activate the expression of angiogenic genes like angiopoietins and vascular endothelial growth factor (VEGF), which may promote angiogenesis and vascular development [1322262732]. Our previous study observed that a hypoxic condition has a potentially novel role in increasing blood vessel density in proximal tibial growth plates through upgulating the HIF-1α/VEGF/VEGFR signalling pathway, contributes to strengthen and enhance the size of the growth plates [67]. Shapiro [19] also indicated that maintenance and synthesis of bone are reliant on the blood supply. In addition, we observed that TBCs in a natural hypoxic condition have more significant upregulation of HIF-1α, -2α, and -3α than AACs in a normoxic condition. Therefore, the absence of reported TD cases in TBCs reared in a high altitude hypoxic environment may be associated with upregulation of HIF-1α, -2α, and -3α.
To study the molecular mechanism of poultry TD further, thiram-induced poultry TD was produced in TBCs. The results were interesting and showed the expression levels of HIF-1α, -2α, and -3α significantly decreased during the thiram treatment phase (days 4–7). During this period, there was 61.7% TD morbidity in TBCs. Subsequently, the percentage of TD morbidity in TBCs declined and HIF expression levels increased on days 10 and 14 when the thiram was removed. These results demonstrated that the expression of HIF-1α, -2α, and -3α during tibial growth in TBCs was inhibited by using thiram. In a similar study reported by Wu et al. [24], there was inhibition of hypoxia-induced retinal angiogenesis by specnuezhenide during suppression of the HIF-1α/VEGF signaling pathway. Another study by Yang et al. [27] showed that silver nanoparticles reduce the roles of HIF-1, VEGF, and glucose transporter type 1 (GLUT1), contributing to the inhibition of cancer cell growth and angiogenesis. The present study results indicate that thiram inhibits the expression levels of HIF-1α, -2α, and -3α in chondrocytes; possibly contributing to the inhibition of angiogenesis in the tibial growth plate [8], which closely associated with the presence of TD.
Simultaneously, to investigate the molecular mechanisms responsible for HIF-1α, -2α, and -3α effects of thiram in commercial broiler chickens (such as AACs), which are the most prone to TD [1518], we tested the effects of thiram on HIF-1α, -2α, and -3α expression in tibial growth plate of AACs. The results indicated that thiram inhibited HIF-1α, -2α, and -3α expression in the tibial growth plate of AACs reared in a high altitude hypoxic environment, and the expression changes were more obvious than those in TBCs, suggesting that HIF-1α, -2α, and -3α are involved in ameliorating the enlargement of tibial growth plate and chondrocyte differentiation under hypoxia [8]. This observation supports the results in a report by Stewart et al. [20], in which they observed growth plate chondrocytes exposed to a hypoxic environment and reported that HIF-2α is probably engaged in the development of blood vessels and suggested that a metabolic shift in growth plate progression was essential for endochondral ossification. In addition, the endurance factor for chondrocytes in the growth plate can be affected by enhanced VEGF expression via HIF-1α dependent mechanisms [17]. It is suggested that upregulation of HIF-1α, -2α, and -3α expression levels in chondrocytes is a protective factor against thiraminduced TD. In this study, the percentage of thiram-induced TD morbidity in AACs was higher than that in TBCs; thus, TBCs have better tolerance to thiram.
In summary, the results have demonstrated that absence of TD in TBCs is closely connected with the upregulation of HIF-1α, -2α, and -3α expression levels in the tibial growth plate under hypoxic conditions. In addition, our thiram-induced poultry TD experiment showed that suppression of HIF-1α, -2α, and -3α expression can possibly contribute to inhibition of angiogenesis in the tibial growth plate. However, description of the associated molecular mechanism(s) remains incomplete. Results of the present study indicate probable mechanisms that explain the association of HIF-1α, -2α, and -3α expression with poultry TD. However, further studies are needed to clarify the expression of the target genes of HIF-1α, -2α, and -3α such as angiopoietins and VEGF and its receptors [322263034], which may be responsible for vessel formation and contribute to tibial growth plate chondrocyte survival against thiram-induced TD.
Figures and Tables
Fig. 1
Natural hypoxic environmental condition elevated hypoxia-inducible factors (HIFs) expression in Tibetan chickens. Protein expression level of HIF-1α (A) was determined by western blotting; RNA expression levels of HIF-1α (B), HIF-2α (C), and HIF-3α (D) were determined by quantitative reverse transcriptase polymerase chain reaction. RNA levels from the normoxia group were normalized to a value of 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control. The results are representative of at least three independent experiments run in triplicate and expressed as means ± SD (n = 3). *p < 0.05, ***p < 0.001.
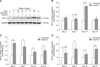
Fig. 2
Upregulation of hypoxia-inducible factor (HIF) expression in Tibetan chickens (TBCs) compared with Arbor Acres chickens (AACs) reared at high altitude. Protein expression level of HIF-1α (A) was determined by western blotting; RNA expression levels of HIF-1α (B), HIF-2α (C), and HIF-3α (D) were determined by quantitative reverse transcriptase polymerase chain reaction. RNA levels from the normoxia group were normalized to a value of 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control. The results are representative of at least three independent experiments run in triplicate and expressed as means ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001.
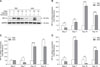
Fig. 3
Thiram inhibited hypoxia-inducible factor (HIF) expression in Tibetan chickens under normoxic conditions. Protein expression level of HIF-1α (A) was determined by western blotting; RNA expression levels of HIF-1α (B), HIF-2α (C), and HIF-3α (D) were determined by quantitative reverse transcriptase polymerase chain reaction. RNA levels from the normoxia group were normalized to a value of 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control. The results are representative of at least three independent experiments run in triplicate and expressed as means ± SD (n = 3). n.s., no significant difference. *p < 0.05, **p < 0.01, ***p < 0.001.
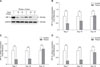
Fig. 4
Thiram inhibited hypoxia-inducible factor (HIF) expression in Arbor Acres chickens reared at high altitude under normoxic conditions. Protein expression level of HIF-1α (A) was determined by western blotting; RNA expression levels of HIF-1α (B), HIF-2α (C), and HIF-3α (D) were determined by quantitative reverse transcriptase polymerase chain reaction. RNA levels from the normoxia group were normalized to a value of 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the loading control. The results are representative of at least three independent experiments run in triplicate and expressed as means ± SD (n = 3). n.s., no significant difference. *p < 0.05, **p < 0.01, ***p < 0.001.
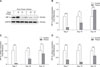
Table 2
Thiram-induced TD morbidity of AACs and TBCs

The percentage of tibial dyschondroplasia (TD) morbidity was determined during days 4 to 7 in thiram-treated Tibetan chickens (TBCs) and Arbor Acres chickens (AACs). The results imply that TBCs have better tolerance to thiram due to being reared at high altitude hypoxic environment. OR, odds ratio; 95% CI, 95% confidence interval.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (No. 31460682). The authors wish to acknowledge and thank the Tibet Agriculture and Animal Husbandry College for providing the test site.
References
1. Befani CD, Vlachostergios PJ, Hatzidaki E, Patrikidou A, Bonanou S, Simos G, Papandreou CN, Liakos P. Bortezomib represses HIF-1α protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J Mol Med (Berl). 2012; 90:45–54.

2. Bohensky J, Terkhorn SP, Freeman TA, Adams CS, Garcia JA, Shapiro IM, Srinivas V. Regulation of autophagy in human and murine cartilage: hypoxia-inducible factor 2 suppresses chondrocyte autophagy. Arthritis Rheum. 2009; 60:1406–1415.

3. Chen J, Chen AY, Huang H, Ye X, Rollyson WD, Perry HE, Brown KC, Rojanasakul Y, Rankin GO, Dasgupta P, Chen YC. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. Int J Oncol. 2015; 46:2629–2638.

4. Cheng KJ, Bao YY, Zhou SH. The role of hypoxia inducible factor in nasal inflammations. Eur Rev Med Pharmacol Sci. 2016; 20:5067–5076.
5. Groves PJ, Muir WI. Earlier hatching time predisposes Cobb broiler chickens to tibial dyschondroplasia. Animal. 2017; 11:112–120.

6. Huang S, Zhang L, Rehman MU, Iqbal MK, Lan Y, Mehmood K, Zhang H, Qiu G, Nabi F, Yao W, Wang M, Li J. High altitude hypoxia as a factor that promotes tibial growth plate development in broiler chickens. PLoS One. 2017; 12:e0173698.

7. Huang SC, Rehman MU, Lan YF, Qiu G, Zhang H, Iqbal MK, Luo HQ, Mehmood K, Zhang LH, Li JK. Tibial dyschondroplasia is highly associated with suppression of tibial angiogenesis through regulating the HIF-1α/VEGF/VEGFR signaling pathway in chickens. Sci Rep. 2017; 7:9089.

8. Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005; 75:200–212.

9. Li H, Guo S, Ren Y, Wang D, Yu H, Li W, Zhao X, Chang Z. VEGF189 expression is highly related to adaptation of the plateau pika (Ochotona curzoniae) inhabiting high altitudes. High Alt Med Biol. 2013; 14:395–404.

10. Li J, Bi D, Pan S, Zhang Y. Effect of diet with thiram on liver antioxidant capacity and tibial dyschondroplasia in broilers. Br Poult Sci. 2007; 48:724–728.

11. Li L, Qu Y, Jin X, Guo XQ, Wang Y, Qi L, Yang J, Zhang P, Li LZ. Protective effect of salidroside against bone loss via hypoxia-inducible factor-1α pathway-induced angiogenesis. Sci Rep. 2016; 6:32131.

12. Li M, Zhao C. Study on Tibetan Chicken embryonic adaptability to chronic hypoxia by revealing differential gene expression in heart tissue. Sci China C Life Sci. 2009; 52:284–295.

13. Markway BD, Cho H, Zilberman-Rudenko J, Holden P, McAlinden A, Johnstone B. Hypoxia-inducible factor 3-alpha expression is associated with the stable chondrocyte phenotype. J Orthop Res. 2015; 33:1561–1570.

14. Nabi F, Shahzad M, Liu J, Li K, Han Z, Zhang D, Iqbal MK, Li J. Hsp90 inhibitor celastrol reinstates growth plate angiogenesis in thiram-induced tibial dyschondroplasia. Avian Pathol. 2016; 45:187–193.

15. Pelicia K, Aparecido Jr IM, Garcia EA, Molino AB, Santos GC, Berto DA, Vieira-Filho JA, Murakami ESM, Montenegro AT, Silva AM. Evaluation of a radiographic method to detect tibial dyschondroplasia lesions in broilers. Braz J Poultry Sci. 2012; 14:129–135.

16. Rath NC, Kannan L, Pillai PB, Huff WE, Huff GR, Horst RL, Emmert JL. Evaluation of the efficacy of vitamin D3 or its metabolites on thiram-induced tibial dyschondroplasia in chickens. Res Vet Sci. 2007; 83:244–250.

17. Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001; 15:2865–2876.

18. Shahzad M, Gao J, Qin P, Liu J, Wang Z, Zhang D, Li J. Expression of genes encoding matrilin-3 and cyclin-I during the impairment and recovery of chicken growth plate in tibial dyschondroplasia. Avian Dis. 2014; 58:468–473.

19. Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008; 15:53–76.

20. Stewart AJ, Houston B, Farquharson C. Elevated expression of hypoxia inducible factor-2alpha in terminally differentiating growth plate chondrocytes. J Cell Physiol. 2006; 206:435–440.

21. Su Y, Li D, Gaur U, Wang Y, Wu N, Chen B, Xu Z, Yin H, Hu Y, Zhu Q. Genetic diversity of bitter taste receptor gene family in Sichuan domestic and Tibetan chicken populations. J Genet. 2016; 95:675–681.

22. Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y. Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44:393–402.

23. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995; 92:5510–5514.

24. Wu J, Ke X, Fu W, Gao X, Zhang H, Wang W, Ma N, Zhao M, Hao X, Zhang Z. Inhibition of hypoxia-induced retinal angiogenesis by specnuezhenide, an effective constituent of Ligustrum lucidum Ait., through suppression of the HIF-1α/VEGF signaling pathway. Molecules. 2016; 21:E1756.
25. Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013; 145:831–841.

26. Yamakawa M, Liu LX, Belanger AJ, Date T, Kuriyama T, Goldberg MA, Cheng SH, Gregory RJ, Jiang C. Expression of angiopoietins in renal epithelial and clear cell carcinoma cells: regulation by hypoxia and participation in angiogenesis. Am J Physiol Renal Physiol. 2004; 287:F649–F657.

27. Yang T, Yao Q, Cao F, Liu Q, Liu B, Wang XH. Silver nanoparticles inhibit the function of hypoxia-inducible factor-1 and target genes: insight into the cytotoxicity and antiangiogenesis. Int J Nanomedicine. 2016; 11:6679–6692.

28. Yang YZ, Bai Y, Dong XX, Zhang JB, Fang M. Expression analysis of HIF-1α and HIF-2α genes in tibetan chicken under normoxia and hypoxia. J Anim Vet Adv. 2012; 10:2172–2175.

29. Yin R, Yuan L, Ping L, Hu L. Neonatal bronchopulmonary dysplasia increases neuronal apoptosis in the hippocampus through the HIF-1α and p53 pathways. Respir Physiol Neurobiol. 2016; 220:81–87.

30. Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004; 131:2161–2171.

31. Zhang C, Li Y, Cornelia R, Swisher S, Kim H. Regulation of VEGF expression by HIF-1α in the femoral head cartilage following ischemia osteonecrosis. Sci Rep. 2012; 2:650.

32. Zhang J, Zhu L, Fang J, Ge Z, Li X. LRG1 modulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via HIF-1α activation. J Exp Clin Cancer Res. 2016; 35:29.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download