Abstract
The use of artificial insemination (AI) in buffalo (Bubalus bubalis) is limited by poor ovarian activity during the hot season, seasonal qualitative patterns in semen, low resistance of sperm cells in the female tract, difficulties in estrus detection, and variable estrus duration. Although AI procedures are commonly used in bovine, use of AI has been limited in buffalo. In the zootechnical field, different studies have been conducted to develop techniques for improvement of fertilizing ability of buffalo spermatozoa after AI. In this study, for the first time, the use of alginate encapsulation and cryopreservation of buffalo spermatozoa is described, and the same procedure was performed with Holstein Friesian (Bos taurus) semen. Results obtained from in vitro analyses indicate that the encapsulation process does not have detrimental effects (compared to controls) on quality parameters (membrane integrity, progressive motility, path average velocity) in either species. Similarly, there were no detrimental effects after cryopreservation in either species. The fertilizing potential of encapsulated and cryopreserved semen was evaluated after AI in 25 buffalo and 113 bovine females. Pregnancy rates were not affected in either species. The results of this study show proof of concept for the use of frozen semen controlled-release devices in buffalo.
Reproductive technologies and biotechnologies are widely used in veterinary and zootechnical fields. In particular, artificial insemination (AI) improves genetic diffusion by partitioning an ejaculate into multiple doses, thereby reducing the number of males and enhancing the scheduling of insemination interventions and health conditions in the farm [1]. During the last 50 years, AI techniques in bovine species have been developed and standardized, as have other procedures including semen extension, cooling, freezing, and thawing [24]. AI is currently performed through the transcervical route and involves placement in the uterine body of approximately 20 million spermatozoa, most of which are phagocytized by leukocytes or removed by the backflow action of the uterus [19]. Compared to natural mating, bovine AI with cooled semen guarantees similar fertility results (> 80%), whereas AI with frozen semen reduces fertility to approximately 60% [2427]. Additionally, the number of spermatozoa used for AI affects the fertilization rate, and a current trend aims to reduce the number of spermatozoa while maintaining high pregnancy and parturition rates [3]. AI with low doses of bovine spermatozoa, including those recovered by sex sorting [10], is typically performed in the uterine horns and has produced promising results [25].
Although AI procedures are commonly used in bovine breeding, these techniques have been limited in buffalo (Bubalus bubalis) [5] because of poor ovarian activity during the hot season, long period between two consecutive births, late maturity of females [28], and difficulties in detecting estrus in a species with a variable estrus duration [17]; all of which combine to reduce female productivity in buffalo. In particular, buffalo estrus duration ranges from 5 to 27 h, and ovulation occurs 6 to 21 h after estrus ends [22]. Moreover, buffalo often exhibit 1 or 2 non-ovulatory follicular waves, followed by an ovulatory one. Non ovulatory and ovulatory follicles have approximately identical diameters [4], thus echographic-based ovulation diagnosis is unreliable. Furthermore, buffalo estrus signs are generally less evident than those in bovine females.
Some strategies, such as ovulation synchronization, prostaglandin or progesterone administration to control the luteal phase, or hormone combinations to control follicular development and ovulation, can limit the effects of these difficulties [5]. Additional problems relate to the short lifespan of frozen/thawed spermatozoa in the female reproductive tract and the narrow uterine cervix of buffalo females which makes intrauterine artificial semen delivery difficult [21].
Some of these obstacles could be overcome by adopting a sperm encapsulation technique, which was previously demonstrated in bovine [13171836] and swine [78232930313334] species with promising results. Alginate encapsulation of sperm can limit damage to spermatozoa and allow prolonged sperm release; moreover, the bioadhesiveness of alginate can prevent semen backflow. To our knowledge, the sperm encapsulation technique has not been applied in buffalo, and the freeze/thaw process for encapsulated spermatozoa has not been optimized. Combining an encapsulation technique with cryopreservation could optimize the use and yield of buffalo semen.
The aim of this work was to provide proof of concept of effective use of frozen semen controlled-release devices when breeding of Mediterranean Italian water buffalo. In vitro analyses (membrane integrity, progressive motility, path average velocity) of semen were performed, and the fertilizing potential of encapsulated and cryopreserved semen was evaluated after AI.
Eight ejaculates from six Mediterranean Italian water buffalo (B. bubalis) and eight ejaculates from seven Holstein Friesian bulls (Bos taurus) of proven fertility were collected (May–July) by using an artificial vagina (semen collection interval range 7–20 days). All subjects were healthy, as verified by regular clinical objective examinations, and reared under a natural photoperiod at an Italian breeding farm. After collection, each ejaculate was diluted 1:1 in a solution of BULLXcell commercial extender (IMV Technologies, France). BULLXcell is a concentrated semen medium used to freeze bovine and buffalo semen and is composed of Tris, citric acid, sugars, glycerol as a penetrating cryoprotectant, ultra-pure water, and antibiotics. Before using the BULLXcell extender solution, it was diluted with three volumes of pure water and one volume of 22% egg yolk-Tris. After dilution, the ejaculate (batch) was characterized based on concentration, plasma membrane integrity, and motility of the spermatozoa and then divided into two aliquots (each aliquot was diluted to obtain a dose of 20 million spermatozoa in 0.5 mL). Samples were placed in straws with 20 million spermatozoa per straw. The first aliquot was frozen, and the second aliquot was encapsulated before freezing. All procedures were performed at room temperature. For each species (bovine and buffalo), four different spermatozoan groups were created: diluted spermatozoa, used as the control (Cbov and Cbuf for bovine and buffalo, respectively), diluted and encapsulated spermatozoa (Ebov and Ebuf for bovine and buffalo, respectively) and, after the freezing and thawing process, diluted frozen/thawed spermatozoa (FT-Cbov and FT-Cbuf) and diluted and encapsulated frozen/thawed spermatozoa (FT-Ebov and FT-Ebuf). The AI were performed in bovine and buffalo females to evaluate the fertility potentials of FT-Cbov, FT-Cbuf, FT-Ebov, and FT-Ebuf semen (Fig. 1).
For each batch, a spermatozoa suspension (diluted with BULLXcell) was added to a sodium alginate solution (1% w/v) (Sigma-Aldrich, Italy). The alginate–sperm suspension was then added drop-wise with a needle (32 G) into a continuously stirred BULLXcell-egg yolk solution containing BaCl2 at 50 mM (CARLO ERBA Reagents, Italy). The barium ions diffused into the droplets, reacted with the alginate chains, and formed alginate gel beads. After 10 min, beads were collected by filtration through a nylon mesh cell strainer (Falcon 100 µm Cell Strainer; Corning, USA) with a 100 µm pore size, rinsed twice with BULLXcell-egg yolk extender solution, and resuspended in the same solution. The 0.5 mL straws were then filled with the bead suspension by using a vacuum pump. The final suspension volume was calculated by using the following equation: where Sv = final suspension volume (mL), N = number of extruded spermatozoa (in millions), V = total straw volume (mL), and 20 = factor used to obtain 20 million spermatozoa per dose.
For size determination, a sample of 200 beads was photographed by using a digital video camera connected to an image analyzer (CV9000 ver. 4.0; FKV, Italy), and the mean diameter was determined.
The spermatozoa-containing straws were equilibrated for 3 h at 4℃ and then frozen using an automatic Mini Digitcool freezer (IMV Technologies). The bovine straw-freezing program included the following steps: +4℃ to −10℃ (rate: −5℃/min), −10℃ to −100℃ (rate: −30℃/min), and −100℃ to −140℃ (rate, −20℃/min). The buffalo straw-freezing program included the following steps: +4℃ to −10℃ (rate, −3℃/min), −10℃ to −110℃ (rate, −40℃/min) and −100℃ to −140℃ (rate, −20℃/min). When the freezer reached −140℃, the frozen straws were immediately plunged and stored in liquid nitrogen.
For the thawing process, two straws from the same frozen batch were thawed for 1 min in a 37℃ water bath (DC5 Haake; ENCO, Italy), pooled in a single tube, and incubated at the same temperature for 14 min.
After the freezing and thawing process, the FT-Cbov, FT-Cbuf, FT-Ebov, and FT-Ebuf spermatozoa samples were analyzed. The diluted spermatozoa samples were characterized after incubation in a 37℃ water bath for 14 min, whereas the encapsulated spermatozoa samples were characterized after dissolution of the beads in an isotonic 3% v/v sodium citrate and 5% v/v EDTA-saline solution (pH 7.4) for approximately 10 min at 37℃, as previously described by Nebel et al. [18].
Aliquots from one tube (containing two straws from the same sample) were used to determine the total cell concentration and plasma membrane integrity (MI) by using an integrated fluorescence microscope (NucleoCounter SP-100; ChemoMetec, Denmark). Briefly, the samples were loaded into a SP1-Cassette and propidium iodide that was immobilized in the SP1-Cassette diffused into cells that had lost MI and stained their DNA. An integrated computer performed the image analysis and counted the dead sperm cells in the sample. The total number of cells was determined by using a lysis buffer (Reagent S100; ChemoMetec) to disrupt the plasma membrane of the sperm cells, thus rendering the nuclei susceptible to staining with propidium iodide. The MI percentage was calculated by using the following equation:
The total motility (TM), progressive motility (PM), and average path velocity (VAP) were determined by a computerized image analyzer (CASA system; Hamilton Thorne, USA) with an HTM-IVOS instrument v.12.3 (Hamilton Thorne). Ten microliters of semen from each tube was placed in two Makler counting chambers (Sefi-Medical Instruments, Israel) at 37℃. Spermatozoon motility was determined for at least 200 cells per sample present in a minimum of four microscopic fields. Spermatozoa with a VAP > 25 µm/sec were defined as motile, whereas sperm with a straight-line velocity/path velocity > 0.8 were defined as progressively motile [37].
The AI were performed between November 2012 and March 2013, a period selected because it is the best buffalo and bovine reproductive season in Italy. For this study, 25 Italian Mediterranean buffalo and 113 Holstein Friesian bovine females were used. Buffalo AI were conducted on one farm, whereas bovine AI were conducted on two different farms.
Estrus synchronization was induced by applying the Ovsynch protocol [611]. Briefly, the animals were synchronized by using a sequence of GnRH (buserelin acetate, 12 mg, Receptal; Intervet, Italy) on day 0 followed by PGF2α (cloprostenol sodium, 524 mg, Estrumate; Intervet) on day 7 and an identical GnRH treatment on day 9. The buffalo were inseminated once at a fixed time (16 h after the second GnRH treatment) and in the body of the uterus by using conventional AI. The AI with FT-Cbuf and FT-Ebuf semen at 20 million sperm per dose were performed by using one straw per insemination. To facilitate the field use of random doses, the straws were stored two-by-two inside a canister, so that a blind selection procedure was used. Pregnancy was diagnosed by ultrasonic examination 60 days after AI and was coded as a binomial event (1, pregnant; 0, not pregnant). All field activities were conducted by one veterinarian.
Two dairy farms (A and B) were involved in this study. On farm A, inseminations were performed after estrus synchronization by applying the Ovsynch protocol. On farm B, AI was performed without applying the synchronization protocol. Inseminations with FT-Cbov and FT-Ebov semen at 20 million sperm per dose were performed by using one straw per insemination. Pregnancy was diagnosed through ultrasonic examination 60 days after insemination, and pregnancy was coded as a binomial event (1, pregnant; 0, not pregnant). All field activities were conducted by one veterinarian at each farm.
The MI, TM, PM, and VAP values were analyzed by using two-way repeated-measures ANOVA with species and treatment (encapsulation and/or cryopreservation) as fixed factors and semen collection time as a repeated-measures variable. One-way ANOVA was performed followed by Tukey's multiple comparison test to assess the differences between treatment groups for sperm characterization. The coefficient of variation (CV) was calculated to evaluate the homogeneous loading of samples in straws. A chi-square test was performed to evaluate the significance of differences among pregnancy rates with FT-C and FT-E semen. Statistical analyses were performed by using SAS 9.1 software (SAS Institute, USA), and the significance level was p < 0.05.
Average bead diameter, determined by optical microscopy and based on a sample of 200 beads, was 2.31 ± 0.15 mm, which is suitable for loading in a 2.5 mm diameter straw. After loading, the mean numbers of non-encapsulated spermatozoa were 18.30 ± 2.32 million/straw (bovine) and 18.42 ± 3.75 million/straw (buffalo), whereas the mean numbers of encapsulated spermatozoa were 23.00 ± 8.00 (bovine) and 20.37 ± 2.49 million/straw (buffalo). In bovines, the CV, a metric of homogeneous straw loading, was lower for FT-Cbov (12.70, n = 30) than for FT-Ebov (37.72, n = 29). However, for buffalo, the FT-Ebuf presented a CV of 12.22 (n = 32), whereas the FT-Cbuf had a CV of 20.37 (n = 33).
A significant treatment effect (p < 0.05) was observed in MI values, whereas the species effect was borderline significant (p = 0.064), most likely due to high variability in the data (panels A in Figs. 2 and 3). For each treatment group, the mean MI values for bovine spermatozoa were higher than those for buffalo sperm cells. The freezing/thawing process reduced MI in the semen of both bovines and buffaloes. No significant differences were observed between the control and encapsulated groups in either species, either before or after cryopreservation.
Significant TM differences were observed among the treatment groups (p < 0.0001), but there was no obvious species effect (p = 0.087). A reduction in TM in both species was observed after encapsulation or cryopreservation (panel C in Figs. 2 and 3). Our results show that bovine spermatozoa were more sensitive to cryopreservation than buffalo spermatozoa were. In fact, after freezing and thawing, diluted spermatozoa lost approximately 44% of their TM, whereas the diluted-encapsulated sperm lost approximately 38% of their TM. The diluted and diluted-encapsulated buffalo spermatozoa lost approximately 23 and 16% of their TM, respectively.
A treatment effect (p < 0.0001), but no species effect (p = 0.22), was noted in PM (panels B in Figs. 2 and 3). The freeze/thaw process significantly reduced PM in both species. Surprisingly, the encapsulated bovine spermatozoa presented the highest PM (panel B in Fig. 2). However, both treatment and species had an influence on VAP (p < 0.001). In particular, VAP values for the bovine spermatozoa were higher than those for the buffalo sperm cells (p < 0.001). In bovines, the encapsulation process slightly increased the VAP (panel D in Fig. 2), whereas, in buffalo, the VAP of the Ebuf group was higher than that of the Cbuf (79.90 ± 28.93 µm/sec and 73.39 ± 25.94 µm/sec, respectively). Cryopreservation significantly (p < 0.05) reduced the VAP of buffalo spermatozoa (60.77 ± 15.52 µm/sec and 56.54 ± 25.92 µm/sec for FT-Cbuf and FT-Ebuf groups, respectively) (panel D in Fig. 3).
Pregnancy rates following AI in female buffalo are reported in Table 1. Although the pregnancy rate obtained with encapsulated spermatozoa (FT-Ebuf group) was higher than that obtained with conventional semen (FT-Cbuf), no significant differences were detected between the two treatment groups (p = 0.087).
The results obtained after AI in bovine females are reported in Table 2. Chi-square test results did not show significant differences in pregnancy rates between the FT-Cbov and FT-Ebov groups for either farm A or farm B. Moreover, the chi-square analysis performed on the pooled results, obtained by the addition of pregnant and non-pregnant individuals at both farms, did not indicate significant differences between the FT-Cbov and FT-Ebov groups. All buffalo and bovine females maintained good health after parturition and newborn weights were not different (p > 0.05) between FT-C and FT-E.
Alginate encapsulation of buffalo spermatozoa is a promising technique for prolonging in utero cell release and preventing semen backflow during AI. However, the technology has not reached an industrial scale, and encapsulated spermatozoa are not commercially available. Moreover, there is no standard method for cryopreservation of encapsulated buffalo semen, even though the availability of frozen semen is important to the widespread application of AI in buffalo. In the present study, an encapsulation and cryopreservation method is proposed for bovine and buffalo spermatozoa, and the quality of the frozen semen was evaluated.
The results obtained from in vitro semen analyses indicate that the encapsulation process does not lead to a decrease in semen quality either before or after cryopreservation in bovines or buffalo. Moreover, the qualitative pattern observed in buffalo semen was similar to that observed in bovines, although the recorded qualitative parameter results were somewhat lower. The percentage of motile bovine spermatozoa was decreased by the encapsulation process (82.10 ± 7.65 vs. 70.93 ± 15.62% for Cbov and Ebov, respectively); however, Vishwanath et al. [35] observed that bovine spermatozoa did not have reduced TM after encapsulation in poly-L-lysine.
In buffalo, the values of MI, TM and PM observed in our study were generally lower than those reported in the literature, which could be related to season of semen collection (May–July); in fact, the low semen quality, during summer season (May–June) has been also reported by Bahga and Khokar [2]. With regard to MI, Leite et al. [15] and Forero-Gonzalez et al. [9] cryopreserved bovine spermatozoa and observed approximately 20% lower MI values than that observed in our study. For buffalo, percentages of MI have been reduced after undergoing a freezing/thawing procedure [1416]. In the present study, the MI in buffalo spermatozoa was lower than that reported in previous studies, but that may be due to high variability of semen quality during hot seasons as indicated by the large standard deviation in our study. In addition, cryopreservation reduces semen quality as measured by PM and VAP, as reported by Orgal et al. [20] for bovines.
Because in vitro semen characterization does not predict the fertilizing potential of spermatozoa with complete reliability, we conducted in vivo AI in both bovines and buffalo. In buffalo, the pregnancy rates were higher for encapsulated spermatozoa (FT-Ebuf) compared to that in the control group (FT-Cbuf); however, there was no significant differences between the two groups.
AI in bovines produced results similar to those in buffalo. The pregnancy rate obtained in synchronized bovine females was not significantly different between encapsulated and nonencapsulated semen. In 1997, Vishwanath et al. [35] conducted an in vivo trial to evaluate the fertilizing properties of bovine spermatozoa encapsulated in an alginate-poly-L-lysine matrix. For that study, heifers were synchronized with a CIDR B device and underwent AI with encapsulated or non-encapsulated spermatozoa. Their pregnancy rate (45%) was similar to that in our study (40%). Recently, Standerholen et al. [32], used 85 bovine semen doses, immobilized in an alginate-based matrix, for an in vivo trial. The authors concluded that there were no differences between the outcome of AI using encapsulated or conventionally diluted frozen sperm.
In addition, we conducted in vivo AI in bovines without hormonal synchronization treatment, and their pregnancy rate was lower than that in synchronized animals; however, there was no difference between the encapsulated and control groups. Many researchers have investigated the effect of freezing on encapsulated mammalian cells, but to our knowledge, only Herrler et al. [12], for humans and bovines, and Shah et al. [26], for dogs, have applied similar techniques to spermatozoa.
The world's critical economic situation requires competent management practices to increase the productivity of buffalo farms. Excellent reproductive efficiency is essential to increase net returns. The use of AI technologies has become important, particularly to improving the genetic health of buffalo herds. However, the time and effort required to detect estrus in buffalo have limited the extensive application and success of technological advances in AI in this species. The results of this study shows proof of concept of using frozen semen controlled-release devices for efficient AI in Mediterranean Italian water buffalo. In our opinion, the results represent an encouraging starting point from which to develop further strategies, thus fulfilling the needs of buffalo breeding programs.
Figures and Tables
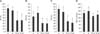 | Fig. 2Bar graphs (means ± SD of eight bovine bulls) of spermatozoa characteristics. (A) membrane integrity (MI), (B) progressive motility (PM), (C) total motility (TM), and (D) average path velocity (VAP) in four bovine treatment groups. C, control spermatozoa; E, encapsulated spermatozoa; FT-C, frozen-thawed control spermatozoa; FT-E, frozen-thawed encapsulated spermatozoa. Values with different letters differ significantly (p < 0.05) among groups. |
 | Fig. 3Bar graphs (means ± SD of eight buffalo bulls) of spermatozoa characteristics. (A) membrane integrity (MI), (B) progressive motility (PM), (C) total motility (TM), and (D) average path velocity (VAP) in four buffalo treatment groups. C, control spermatozoa; E, encapsulated spermatozoa; FT-C, frozen-thawed control spermatozoa; FT-E, frozen-thawed encapsulated spermatozoa. Values with different letters differ significantly (p < 0.05) among groups. |
Acknowledgments
This work was supported by Ministry of Agricultural, Food and Forestry Policies (MiPPAF; Project Det. 13576, May 24th 2011), Italy.
References
1. Amiridis GS, Cseh S. Assisted reproductive technologies in the reproductive management of small ruminants. Anim Reprod Sci. 2012; 130:152–161.

2. Bahga CS, Khokar BS. Effect of different seasons on concentration of plasma luteinizing hormone and seminal quality vis-à-vis freezability of buffalo bulls (Bubalus bubalis). Int J Biometeorol. 1991; 35:222–224.

3. Ballester J, Johannisson A, Saravia F, Håård M, Gustafsson H, Bajramovic D, Rodriguez-Martinez H. Post-thaw viability of bull AI-doses with low-sperm numbers. Theriogenology. 2007; 68:934–943.

4. Baruselli PS, Mucciolo RG, Visintin JA, Viana WG, Arruda RP, Madureira EH, Oiveira CA, Molero-Filho JR. Ovarian follicular dynamics during the estrous cycle in buffalo (Bubalus bubalis). Theriogenology. 1997; 47:1531–1547.

5. De Rensis F, López-Gatius F. Protocols for synchronizing estrus and ovulation in buffalo (Bubalus bubalis): a review. Theriogenology. 2007; 67:209–216.

6. De Rensis F, Ronci G, Guarneri P, Nguyen BX, Presicce GA, Huszenicza G, Scaramuzzi RJ. Conception rate after fixed time insemination following ovsynch protocol with and without progesterone supplementation in cyclic and noncyclic Mediterranean Italian buffaloes (Bubalus bubalis). Theriogenology. 2005; 63:1824–1831.

7. Faustini M, Bucco M, Galeati G, Spinaci M, Villani S, Chlapanidas T, Ghidoni I, Vigo D, Torre ML. Boar sperm encapsulation reduces in vitro polyspermy. Reprod Domest Anim. 2010; 45:359–362.
8. Faustini M, Vigo D, Spinaci M, Galeati G, Torre ML. Enhancing insemination performance in pigs through controlled release of encapsulated spermatozoa. Reprod Domest Anim. 2012; 47:Suppl 4. 353–358.

9. Forero-Gonzalez RA, Celeghini ECC, Raphael CF, Andrade AFC, Bressan FF, Arruda RP. Effects of bovine sperm cryopreservation using different freezing techniques and cryoprotective agents on plasma, acrosomal and mitochondrial membranes. Andrologia. 2012; 44:Suppl 1. 154–159.

10. Garner DL, Seidel GE Jr. History of commercializing sexed semen for cattle. Theriogenology. 2008; 69:886–895.

11. Gaviraghi A, Puglisi R, Balduzzi D, Severgnini A, Bornaghi V, Bongioni G, Frana A, Gandini LM, Lukaj A, Bonacina C, Galli A. Minimum number of spermatozoa per dose in Mediterranean Italian buffalo (Bubalus bubalis) using sexed frozen semen and conventional artificial insemination. Theriogenology. 2013; 79:1171–1176.

12. Herrler A, Eisner S, Bach V, Weissenborn U, Beier HM. Cryopreservation of spermatozoa in alginic acid capsules. Fertil Steril. 2006; 85:208–213.

13. Kemmer C, Fluri DA, Witschi U, Passeraub A, Gutzwiller A, Fussenegger M. A designer network coordinating bovine artificial insemination by ovulation-triggered release of implanted sperms. J Control Release. 2011; 150:23–29.

14. Kumar R, Atreja SK. Effect of incorporation of additives in tris-based egg yolk extender on buffalo (Bubalus bubalis) sperm tyrosine phosphorylation during cryopreservation. Reprod Domest Anim. 2012; 47:485–490.

15. Leite TG, do Vale Filho VR, de Arruda RP, de Andrade AFC, Emerick LL, Zaffalon FG, Martins JAM, de Andrade VJ. Effects of extender and equilibration time on post-thaw motility and membrane integrity of cryopreserved Gyr bull semen evaluated by CASA and flow cytometry. Anim Reprod Sci. 2010; 120:31–38.

16. Minervini F, Guastamacchia R, Pizzi F, Dell’Aquila ME, Barile VL. Assessment of different functional parameters of frozen-thawed buffalo spermatozoa by using cytofluorimetric determinations. Reprod Domest Anim. 2013; 48:317–324.

17. Monteiro BM, de Souza DC, Vasconcellos GSFM, Corrêa TB, Vecchio D, de Sá MF, de Carvalho NAT, Baruselli PS. Ovarian responses of dairy buffalo cows to timed artificial insemination protocol, using new or used progesterone devices, during the breeding season (autumn-winter). Anim Sci J. 2016; 87:13–20.

18. Nebel RL, Bame JH, Saacke RG, Lim F. Microencapsulation of bovine spermatozoa. J Anim Sci. 1985; 60:1631–1639.

19. Nebel RL, Vishwanath R, McMillan WH, Saacke RG. Microencapsulation of bovine spermatozoa for use in artificial insemination: a review. Reprod Fertil Dev. 1993; 5:701–712.

20. Orgal S, Zeron Y, Elior N, Biran D, Friedman E, Druker S, Roth Z. Season-induced changes in bovine sperm motility following a freeze-thaw procedure. J Reprod Dev. 2012; 58:212–218.

23. Perteghella S, Vigani V, Crivelli B, Spinaci M, Galeati G, Bucci D, Vigo D, Torre ML, Chlapanidas T. Sperm encapsulation from 1985 to date: technology evolution and new challenges in swine reproduction. Reprod Domest Anim. 2015; 50:Suppl 2. 98–102.

24. Rodriguez-Martinez H. Assisted reproductive techniques for cattle breeding in developing countries: a critical appraisal of their value and limitations. Reprod Domest Anim. 2012; 47:Suppl 1. 21–26.

25. Seidel GE Jr, Schenk JL. Pregnancy rates in cattle with cryopreserved sexed sperm: effects of sperm numbers per inseminate and site of sperm deposition. Anim Reprod Sci. 2008; 105:129–138.

26. Shah S, Otsuki T, Fujimura C, Yamamoto N, Yamashita Y, Higaki S, Hishinuma M. Cryopreservation of microencapsulated canine sperm. Theriogenology. 2011; 75:679–686.

27. Silva PFN, Gadella BM. Detection of damage in mammalian sperm cells. Theriogenology. 2006; 65:958–978.

28. Singh J, Nanda AS, Adams GP. The reproductive pattern and efficiency of female buffaloes. Anim Reprod Sci. 2000; 60-61:593–604.
29. Spinaci M, Bucci D, Chlapanidas T, Vallorani C, Perteghella S, Communod R, Vigo D, Tamanini C, Galeati G, Faustini M, Torre ML. Boar sperm changes after sorting and encapsulation in barium alginate membranes. Theriogenology. 2013; 80:526–532.

30. Spinaci M, Chlapanidas T, Bucci D, Vallorani C, Perteghella S, Lucconi G, Communod R, Vigo D, Galeati G, Faustini M, Torre ML. Encapsulation of sex sorted boar semen: sperm membrane status and oocyte penetration parameters. Theriogenology. 2013; 79:575–581.

31. Spinaci M, Perteghella S, Chlapanidas T, Galeati G, Vigo D, Tamanini C, Bucci D. Storage of sexed boar spermatozoa: limits and perspectives. Theriogenology. 2016; 85:65–73.

32. Standerholen FB, Waterhouse KE, Larsgard AG, Garmo RT, Myromslien FD, Sunde J, Ropstad E, Klinkenberg G, Kommisrud E. Use of immobilized cryopreserved bovine semen in a blind artificial insemination trial. Theriogenology. 2015; 84:413–420.

33. Vigo D, Faustini M, Torre ML, Pecile A, Villani S, Asti A, Norbert R, Maggi L, Conte U, Cremonesi F, Stacchezzini S, Maffeo G. Boar semen controlled-delivery system: morphological investigation and in vitro fertilization test. Reprod Fertil Dev. 2002; 14:307–314.

34. Vigo D, Faustini M, Villani S, Orsini F, Bucco M, Chlapanidas T, Conte U, Ellis K, Torre ML. Semen controlled-release capsules allow a single artificial insemination in sows. Theriogenology. 2009; 72:439–444.

35. Vishwanath R, Nebel RL, McMillan WH, Pitt CJ, Macmillan KL. Selected times of insemination with microencapsulated bovine spermatozoa affect pregnancy rates of synchronized heifers. Theriogenology. 1997; 48:369–376.

36. Weber W, Rimann M, Schafroth T, Witschi U, Fussenegger M. Design of high-throughput-compatible protocols for microencapsulation, cryopreservation and release of bovine spermatozoa. J Biotechnol. 2006; 123:155–163.

37. World Health Organization. Semen analysis: standard procedures. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO Press;2010. p. 7–115. Chapt. 2.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download