Abstract
This study was performed to identify the relationships between hepatic vein (HV) measurements, including flow velocity and waveform, using pulsed-wave (PW) Doppler ultrasonography, and the severity of tricuspid regurgitation (TR) in dogs. The study included 22 dogs with TR and 7 healthy dogs. The TR group was subdivided into 3 groups according to TR jet profile obtained by echocardiography. The hepatic venous waveform was obtained and classified into 3 types. A variety of HV measurements, including the maximal velocities of the atrial systolic, systolic (S), end ventricular systolic, and diastolic (D) waves and the ratio of the S- and D- wave velocities (S/D ratio), were acquired. TR severity was significantly correlated with the S- (r = −0.380, p = 0.042) and D- (r = 0.468, p = 0.011) wave velocities and the S/D ratio (r = −0.747, p < 0.001). Receiver operating characteristic curve analysis revealed the highest sensitivity and specificity for the S/D ratio (89% and 75%, respectively) at a threshold of 0.97 with excellent accuracy (AUC = 0.911, p < 0.001). In conclusion, PW Doppler ultrasonography of the HV can be used to identify the presence of significant TR and to classify TR severity in dogs.
Doppler ultrasonography is an essential, non-invasive modality for evaluating hepatic vasculature and parenchymal disease in human and veterinary medicine [101116]. This modality provides real-time anatomical information, as well as information on blood flow parameters including velocity, resistivity, and direction [1016]. A previous study showed that accurate interpretation of the hepatic vein (HV) waveform detected with pulsed-wave (PW) Doppler ultrasonography is clinically valuable, as it is greatly influenced by both cardiac and hepatic factors [22].
Several studies have evaluated the normal HV pattern by applying PW Doppler ultrasonography in dogs [52021]. The normal HV waveform is tetraphasic and consists of large antegrade waves (systolic [S]- and diastolic [D]-waves) and small retrograde waves (atrial systolic [A]- and ventricular systolic [V]-waves) that result from variations in the cardiac cycle and right atrial pressure (Fig. 1) [22]. Concurrent electrocardiography (ECG) tracing is used as a timing reference for the HV waveform. The A-wave corresponds to right atrial contraction and occurs after the P-wave in the ECG trace. With atrial contraction, blood is propelled retrograde toward the caudal vena cava and into the HV. Systole is characterized by a prominent antegrade systolic wave (S-wave). The S-wave begins immediately after the QRS complex and develops from movement of the tricuspid valve annulus toward the apex and ventricular wall contraction. These events result in negative pressure in the atrium, causing a large amount of antegrade blood flow toward the heart. The V-wave follows the S-wave and occurs near the end of the T-wave in the ECG trace. This wave can be antegrade or retrograde and corresponds to atrial overfilling, which results from the closed tricuspid valve and its movement toward the normal position. Diastole is characterized by an antegrade diastolic wave (D-wave), which follows the T-wave in the ECG trace and is caused by negative atrial pressure created when blood flows into the relaxing right ventricle (RV). During this period, with ventricular relaxation and the tricuspid valve open, the right atrium (RA) serves as a passive conduit for venous return.
The characteristic tetraphasic waveform of a normal HV is similar in humans and dogs [5202122]. In humans, analysis of the waveform has been shown to be useful in the differential diagnosis of right-sided heart diseases and various liver diseases [22]. The identification of an abnormal waveform has been shown to be sensitive for the detection of tricuspid regurgitation (TR) in patients with ambiguous abdominal symptoms, hepatomegaly, high liver enzyme activities, and ascites [119]. Moreover, the HV waveform, along with various echocardiographic parameters, can be used as a semi-quantitative parameter for grading tricuspid regurgitation (TR) severity [26].
To our knowledge, no previous study has described HV velocity and waveform in dogs with TR. Therefore, the present study aimed to identify the relationships between HV measurements, including flow velocity and waveform performed with PW Doppler ultrasonography, and TR severity in dogs. Furthermore, the study assessed the relationship between the echocardiographic grade of TR severity and the observed types of HV waveforms.
Dogs presenting to the Konkuk Veterinary Medical Teaching Hospital from March 2012 to May 2013 were enrolled in this study. The inclusion criteria were as follows: (1) TR detected through echocardiography; (2) complete echocardiographic reports for classifying TR severity; (3) absence of hepatic disease confirmed by liver enzyme profiles, abdominal ultrasound, and fine needle aspiration; and (4) normal blood pressures, including systolic, diastolic, and mean arterial pressure as assessed by using an oscillometric blood pressure monitor (Cardell Veterinary Monitor 9401; Paragon Medical Supply, USA). The enrolled dogs underwent duplex Doppler ultrasonic examinations of the HV within 24 h of echocardiography. Dogs with significant respiratory movement or a biphasic or monophasic waveform were excluded. Care and maintenance of the dogs and the study design followed protocols approved by the Institutional Animal Care and Use Committee of Konkuk University (approval No. KU13169).
The study included TR and control groups. The TR group included 22 dogs (6 Malteses, 5 Yorkshire Terriers, 4 Pekineses, 2 Shih-tzus, 2 Cocker Spaniels, 2 Schnauzers, and 1 mixed breed). Of the 22 dogs, 7 were castrated males, 2 were intact males, 8 were spayed females, and 5 were intact females. In the TR group, the mean age of the dogs was 11.05 ± 2.24 years (range, 8–17 years), and the mean weight was 5.11 ± 2.47 kg (range, 2.0–11.8 kg).
The control group included 7 randomly selected dogs without cardiac, hepatic, or biliary disease (2 Malteses, 1 Cocker Spaniel, 1 Poodle, 1 Chihuahua, and 1 mixed breed). Of the 7 dogs, 2 were castrated males, 3 were spayed females, and 2 were intact females. In the control group, the mean age of the dogs was 6.57 ± 2.07 years (range, 3–9 years), and the mean weight was 5.26 ± 2.43 kg (range, 2.1–8.6 kg). Health status was evaluated through physical examination, complete blood count, serum chemistry, radiology, ultrasonography, and prior echocardiography. The heartworm antigen test result was negative in all dogs.
Echocardiographic examinations and evaluation of TR severity were performed by a trained echocardiographer (JH Kim) who was blinded to the results of the HV flow pattern analysis. The echocardiographic examinations were performed by using an ultrasonographic machine (Prosound Alpha 6; Aloka, Japan) with a 7 MHz sector transducer. The right parasternal long-axis 4-chamber, left apical 4-chamber, and left parasternal short-axis views were used for evaluating tricuspid valve morphology, dilation of the RA and RV, and color flow TR jet. The left apical 4-chamber view was used for continuous wave (CW) Doppler recording of the TR jet to evaluate signal intensity and contour of the jet flow. All dogs were examined thrice during 3 consecutive cardiac cycles.
According to parameters presented by the American Society of Echocardiography recommendations for assessing valvular regurgitation [26], the TR group was subdivided into mild, moderate, and severe TR groups. Significant TR was defined as moderate to severe TR.
All ultrasonographic examinations were performed by a trained sonographer (SY Kim) who was blinded to the result of echocardiography. Ultrasonographic examinations were performed by using the Prosound Alpha 6 ultrasonographic machine (Aloka) with an 8 MHz microconvex transducer. The dogs were placed in dorsal recumbency for abdominal ultrasonographic examination. ECG leads were attached to the limbs, and cardiac rhythm was displayed simultaneously with the B-mode images and the PW Doppler waveforms.
PW Doppler ultrasonography of the right medial liver lobe or quadrate liver lobe HV was performed via the subcostal approach. These 2 branches were selected to evaluate the HV waveform due to their nearly parallel orientation to the Doppler signal. The HV waveforms were obtained during quiet respiration or at the end of expiration to avoid alteration of the waveforms by various respiratory influences. The waveforms were recorded for at least 3 consecutive cycles by using a 1.5 or 2 mm PW Doppler sample volume placed between the bifurcation of the right medial and quadrate HVs and 2 cm away from the bifurcation (Fig. 2).
Measurements were performed by using electronic calipers and included the directions and maximal velocities of the S-, D-, V-, and A-waves. Three complete waveforms were measured, and the mean value of the maximum segmental velocities was calculated for each velocity peak. The ratio of the S-wave velocity to the D-wave velocity (S/D ratio) was estimated to exclude potential variations in velocities due to slight differences in sample volume placements among the dogs.
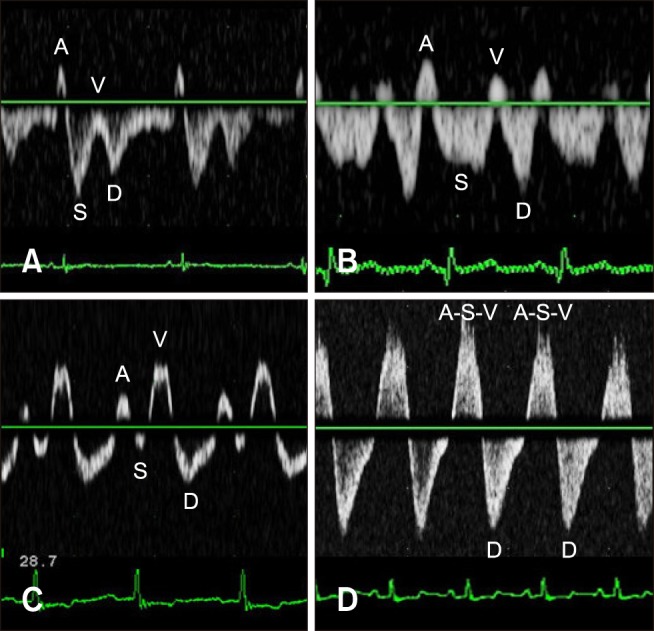
The HV waveforms were classified into the following 3 types (Fig. 3): type 1 [systolic dominance pattern with the S-wave larger than the D-wave (S/D ratio ≥1), resembling a normal pattern]; type 2 [reversed flow pattern with the S-wave smaller than the D-wave and with anterograde flow (0 < S/D ratio < 1)]; and type 3 [retrograde flow (S/D ratio ≤ 0) during ventricular systole and possible fusion of the S-wave with the A- and V-waves].
Differences in the parameters of the HV waveforms (S-, A-, V-, and D-wave velocities and the S/D ratios) between the TR and control groups were assessed by using the Mann-Whitney test. Correlations of the TR grade with S-, A-, V-, and D-wave velocities and the S/D ratio were investigated by using Spearman's rank correlation. Receiver operating characteristic (ROC) curve analysis was used to examine the sensitivities and specificities of various S/D ratio cut-off values for predicting significant TR. The relationships between TR severity according to echocardiographic parameters and the types of HV flow patterns were investigated by using the linear association test. All statistical analyses were performed by using the Statistical Package for the Social Sciences software (SPSS 19.0 for Windows; SPSS, USA), and the criterion for statistical significance was set as p < 0.05.
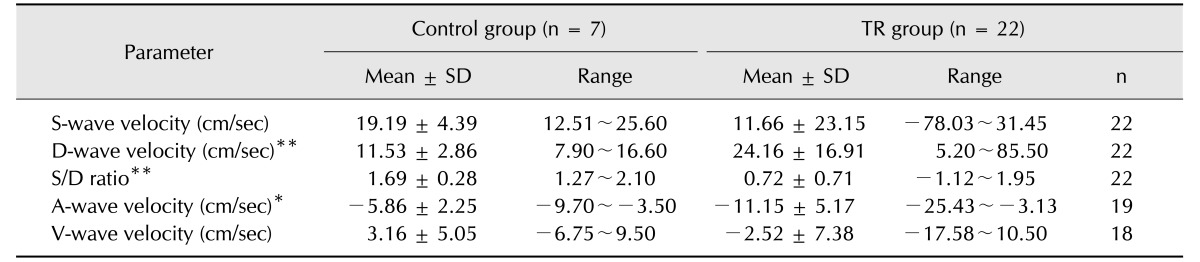
The HV flow velocities of each component (A-, S-, V-, and D-waves) and the S/D ratio in the TR and control groups are presented in Table 1. In the control group, all dogs showed antegrade S- and D-waves, and the S-wave velocity was greater than the D-wave velocity in all dogs. Additionally, the S/D ratio was above 1.27 in all dogs. The A-wave was retrograde in 5 dogs and was absent in 2 dogs, and the V-wave was antegrade in 6 dogs and was retrograde in 1 dog. The HV waveform was classified as type 1 in all dogs in the control group. In the TR group, the S-wave was retrograde in 4 dogs and was antegrade in 18 dogs. The S-wave velocity was smaller than the D-wave velocity in 10 dogs and was greater than the D-wave velocity in 9 dogs. Additionally, the A-wave was absent in 3 dogs and was retrograde in 19 dogs. The V-wave was absent in 4 dogs, was antegrade in 4 dogs, and was retrograde in 14 dogs. The 4 dogs without a V-wave showed retrograde systolic flow. The HV waveform was classified as type 1 in 9 dogs, type 2 in 9 dogs, and type 3 in 4 dogs in the TR group. The mean velocity of the S-wave was lower in the TR group than in the control group; however, the difference was not statistically significant. The S/D ratio and the maximal velocities of the D- and A-waves were significantly smaller in the TR group than in the control group.
The TR severity was mild in 13 dogs, moderate in 6 dogs, and severe in 3 dogs. In the mild TR group, the mean velocities of the S-, D-, A-, and V-waves were 22.38 ± 2.02, 21.22 ± 10.84, −12.71 ± 9.33, and −0.21 ± 3.89 cm/sec, respectively, and the S/D ratio was 1.22 ± 0.49. In the moderate TR group, the mean velocities of the S-, D-, A-, and V-waves were 13.10 ± 12.10, 22.86 ± 10.61, −11.58 ± 5.31, and −6.15 ± 3.49 cm/sec, respectively, and the S/D ratio was 0.56 ± 0.69. In the severe TR group, the mean velocities of the S- and D-waves were −37.70 ± 35.10 and 29.52 ± 40.02 cm/sec, respectively, and the S/D ratio was −1.02 ± 0.11. In this group, the A- and V-waves could not be evaluated due to fusion of the A-, S-, and V-waves (panel D in Fig. 3).
There was a relatively strong negative correlation between TR severity and the S/D ratio (r = −0.747, p < 0.001, r2 = 0.676). Additionally, there was a moderate negative correlations between TR severity and the S-wave velocity (r = −0.380, p = 0.042, r2 = 0.387) and a moderately positive correlation between TR severity and the D-wave velocity (r = 0.468, p = 0.011, r2 = 0.212). Other parameters, such as A- and V-wave velocities, were not significantly correlated with TR severity.
The ROC curve analysis revealed that the highest sensitivity and specificity were for the S/D ratio (89% and 75%, respectively) at a threshold of 0.97 and with excellent accuracy (AUC = 0.911, p < 0.001).
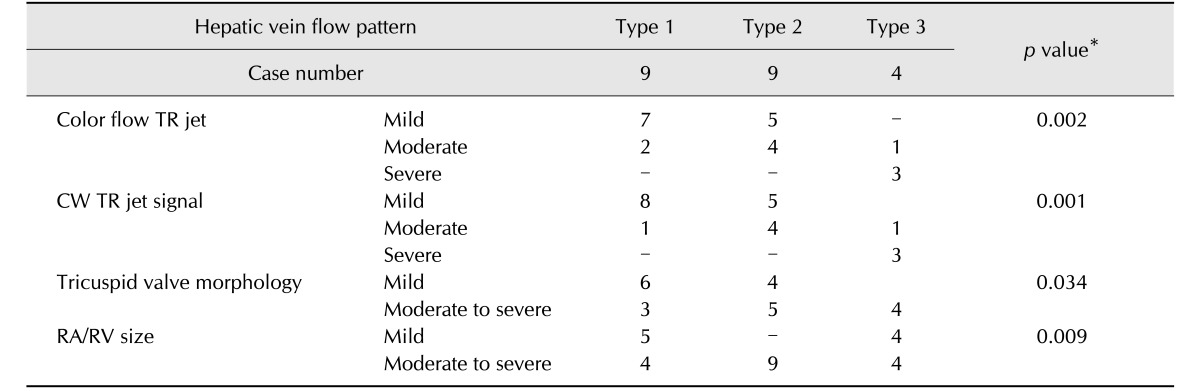
The cross-table of the echocardiographic TR grade according to each type of HV waveform and the p value for each parameter are summarized in Table 2. When HV flow patterns were compared with TR severity, as assessed through echocardiography, mild and moderate TR were mainly associated with types 1 and 2, and severe TR was mainly associated with type 3. In the linear association test, there were statistically significant relationships between echocardiographic parameters (RA/RV size, tricuspid valve morphology, CW TR jet profile, and color flow TR jet) and the types of HV flow patterns.
The present study demonstrated the feasibility of evaluating flow velocities and waveforms of HV by using PW Doppler ultrasonography in the detection of a significant TR and classification of its severity in dogs. The S-wave velocity and S/D ratio decreased and the D-wave velocity increased as TR severity increased. Furthermore, the type of HV flow pattern had a positive relationship with TR severity, as evaluated through echocardiographic parameters.
In previous studies with humans, the S/D ratio was smaller in TR groups than in control groups, and, in most patients with TR, the S/D ratio was smaller than 0.6. In a previous study [1], all patients with TR had a smaller systolic wave velocity than diastolic wave velocity. In another study on 47 patients with TR [6], 30 patients had reverse systolic flow and 12 patients had systolic blunting, and the S/D ratio was smaller than 0.6. In that study, 5 of the 47 patients and 97% of control group had a normal HV flow pattern. Therefore, in reports on humans, most patients with TR had an S/D ratio of less than 0.6. In the present study, the S/D ratio was less than 0.6 (n = 4) or the systolic flow was reversed (n = 10) among 15 dogs with TR. Also, in this study, although the S/D ratio was significantly smaller in the TR group than in the control group, the cut-off value of the S/D ratio for identifying the presence of TR was not clearly demonstrated. Of the dogs in the TR group, 41% had a systolic dominant HV waveform, which is similar to that in the control group. There was considerable overlap of S/D ratios between the two groups; thus, S/D ratio was not sufficient for the diagnosis of TR.
Many normal dogs have trivial to mild TR, and this should be differentiated from moderate to severe TR induced by tricuspid valve disease [2]. Therefore, an S/D ratio cut-off value for diagnosing significant TR should be established. In the present study, an S/D ratio less than 0.97 showed a high sensitivity and specificity for predicting moderate to severe TR and indicates the possibility of the presence of significant TR in dogs.
In our study, as the TR severity increased, the S-wave velocity showed a decreasing pattern while the D-wave velocity showed an increasing pattern, which resulted in a smaller S/D ratio than usual. These characteristics of hepatic venous velocities related with TR have also been recognized in human studies [619]. During ventricular systole under normal conditions, HV flow is characterized as a prominent antegrade wave produced by movement of the tricuspid valve annulus toward the apex and ventricular wall contraction. Conversely, under TR, blood regurgitates retrograde through the tricuspid valve into the RA, caudal vena cava, and HV, resulting in a decreased systolic wave in the HV or, more severely, a retrograde systolic wave in the HV [22]. During ventricular diastole with the tricuspid valve open and myocardium relaxed, blood congested in the RA, CVC, and HVs flows passively into the RV, resulting in a large antegrade D-wave [22].
The type of HV waveform has been used as a parameter to grade TR severity in human study [13]. In that study, patients with a type 1 HV pattern were considered to have mild TR, those with a type 2 pattern were considered to have moderate TR, and those with a type 3 pattern were considered to have severe TR. Another study reported that a type 3 HV waveform had a sensitivity of 80% for detecting severe TR [6]. In the present study, four qualitative echocardiographic parameters were used to decide the correlation in the classification of TR grade between HV pattern and echocardiography. The echocardiographic grade of TR severity showed a statistically significant relationship with the type of HV waveform, except for tricuspid valve morphology, indicating that TR severity could be graded by using HV flow patterns. Furthermore, 3 of the 4 dogs with a type 3 flow pattern were considered to have severe TR according to all echocardiographic parameters, and the type 3 pattern could be used to differentiate severe TR from the other grades of TR in dogs.
Through the subcostal approach, the HVs could be easily evaluated at a very small scan angle in both the control and TR groups. In the control group, features of the normal waveform allowed the identification of each component (tetraphasic waves), even without ECG recording. However, in the TR group, several dogs required simultaneous ECG recording to identify precisely the flow pattern corresponding to the cardiac cycle. In addition, the complete tetraphase in our TR group did not match precisely to that in previous human studies, especially the A- and V-waves. Frequently, A- or V-waves were absent or exaggerated by respiratory movements, causing a marked translational motion artifact, and ECG tracing was necessary to identify the cardiac cycle. Thus, in our opinion, S- and D- wave velocities and the S/D ratio could be used easily for the evaluation of TR severity in dogs. Usually, PW Doppler ultrasonographic examination is easily interrupted by respiratory motion; therefore, identification and evaluation of the HV waveform are difficult when dogs were exhibiting severe panting or an unstable status. In addition, it was more difficult to obtain an accurate image among dogs in the control group than among dogs in the TR group, as the HV was smaller among dogs in the control group than among dogs in the TR group [6].
The results of the present study indicate that PW Doppler ultrasonographic evaluation of the HV can be useful for further evaluation of dogs with right-sided cardiac diseases, such as restrictive cardiomyopathy [18], constrictive pericarditis [23], and pulmonary hypertension [825]. HV waveform alterations have been reported in humans with various hepatic diseases, such as liver fibrosis, diffuse metastatic liver disease, lipidosis, chronic hepatitis, acute viral hepatitis, and cirrhosis [49121415172224]. Therefore, further studies evaluating the HV patterns in dogs with various hepatic and cardiac diseases are required.
The present study has several limitations. First, qualitative parameters were used when creating TR severity subgroups due to the lack of a gold standard for evaluating TR severity. Although right ventriculography has been used to define the degree of regurgitation, it is dependent on hemodynamics and several technical factors. Moreover, it has not been performed in veterinary medicine routinely, and it was difficult to apply in this study. In addition, other quantitative parameters, such as the vena contracta width, the proximal isovelocity surface area method, the effective orifice regurgitant area, and the regurgitant volume were not considered due to the lack of standard values in dogs. Second, there was a large difference in the number of dogs according to TR severity, and this might have influenced the statistical assessment of the significance of the observed differences. Third, pulmonary pressure, right atrial pressure, heart rate, and other hemodynamic factors were not considered in this study. These factors might cause mild alterations of maximal velocities. However, a previous study in dogs reported that the overall appearance of the HV waveforms were relatively consistent in different hemodynamic states, including cardiovascular depression and hyperdynamic circulation [21]. Finally, inter-observer variability was not considered in this study.
In conclusion, PW Doppler ultrasonographic examination of the HV can be used to identify the presence of clinically significant TR in dogs. When the S/D ratio is less than 0.97, significant TR should be considered. Moreover, in addition to various echocardiographic parameters, the type of HV waveform can be used for grading TR severity in dogs.
Acknowledgments
This paper was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2015.
References
1. Abu-Yousef MM. Duplex Doppler sonography of the hepatic vein in tricuspid regurgitation. AJR Am J Roentgenol. 1991; 156:79–83. PMID: 1898574.

2. Boon JA. Acquired valvular disease. In: Veterinary Echocardiography. 2nd ed. Oxford: Wiley-Blackwell;2011. p. 267–334.
3. Coulden RA, Lomas DJ, Farman P, Britton PD. Doppler ultrasound of the hepatic veins: normal appearances. Clin Radiol. 1992; 45:223–227. PMID: 1395374.

4. Colli A, Cocciolo M, Riva C, Martinez E, Prisco A, Pirola M, Bratina G. Abnormalities of Doppler waveform of the hepatic veins in patients with chronic liver disease: correlation with histologic findings. AJR Am J Roentgenol. 1994; 162:833–837. PMID: 8141001.

5. Finn-Bodner ST, Hudson JA. Abdominal vascular sonography. Vet Clin North Am Small Anim Pract. 1998; 28:887–942. PMID: 9698620.

6. Gonzalez-Vilchez F, Zarauza J, Vazquez de Prada JA, Martín Durán R, Ruano J, Delgado C, Figueroa A. Assessment of tricuspid regurgitation by Doppler color flow imaging: angiographic correlation. Int J Cardiol. 1994; 44:275–283. PMID: 8077074.

7. Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000; 45:23–41. PMID: 10802332.

8. Ginghină C, Muraru D, Vlădaia A, Jurcuţ R, Popescu BA, Călin A, Giuşcă S. Doppler flow patterns in the evaluation of pulmonary hypertension. Rom J Intern Med. 2009; 47:109–121. PMID: 20067161.
9. Hamato N, Moriyasu F, Someda H, Nishikawa K, Chiba T, Okuma M. Clinical application of hepatic venous hemodynamics by Doppler ultrasonography in chronic liver disease. Ultrasound Med Biol. 1997; 23:829–835. PMID: 9300986.

10. Koslin DB, Berland LL. Duplex Doppler examination of the liver and portal venous system. J Clin Ultrasound. 1987; 15:675–686. PMID: 3119672.

12. Kok T, van der Jagt EJ, Haagsma EB, Bijleveld CM, Jansen PL, Boeve WJ. The value of Doppler ultrasound in cirrhosis and portal hypertension. Scand J Gastroenterol Suppl. 1999; 230:82–88. PMID: 10499467.
13. Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL. European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010; 11:307–332. PMID: 20435783.

14. Martínez-Noguera A, Montserrat E, Torrubia S, Villalba J. Doppler in hepatic cirrhosis and chronic hepatitis. Semin Ultrasound CT MR. 2002; 23:19–36. PMID: 11866220.

15. Murat A, Akarsu S, Cihangiroğlu M, Yildirim H, Serhatlioğlu S, Kalender O. Assessment of Doppler waveform patterns and flow velocities of hepatic veins in children with acute viral hepatitis. Diagn Interv Radiol. 2006; 12:85–89. PMID: 16752355.
16. Nyland TG, Mattoon JS. Liver. Small Animal Diagnostic Ultrasound. 2nd ed. Philadelphia: WB Saunders;2002. p. 93–127.
17. Oguzkurt L, Yildirim T, Torun D, Tercan F, Kizilkilic O, Niron EA. Hepatic vein Doppler waveform in patients with diffuse fatty infiltration of the liver. Eur J Radiol. 2005; 54:253–257. PMID: 15837406.

18. Oh JK, Seward JB, Tajik AJ. Valvular heart disease. The Echo Manual. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;2006. p. 189–225.
19. Sakai K, Nakamura K, Satomi G, Kondo M, Hirosawa K. Evaluation of tricuspid regurgitation by blood flow pattern in the hepatic vein using pulsed Doppler technique. Am Heart J. 1984; 108:516–523. PMID: 6475714.

20. Szatmári V, Sótonyi P, Vörös K. Normal duplex Doppler waveforms of major abdominal blood vessels in dogs: a review. Vet Radiol Ultrasound. 2001; 42:93–107. PMID: 11327368.
21. Smithenson BT, Mattoon JS, Bonagura JD, Abrahamsen EJ, Drost WT. Pulsed-wave Doppler ultrasonographic evaluation of hepatic veins during variable hemodynamic states in healthy anesthetized dogs. Am J Vet Res. 2004; 65:734–740. PMID: 15198211.

22. Scheinfeld MH, Bilali A, Koenigsberg M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics. 2009; 29:2081–2098. PMID: 19926763.

23. Von Bibra H, Schober K, Jenni R, Busch R, Sebening H, Blömer H. Diagnosis of constrictive pericarditis by pulsed Doppler echocardiography of the hepatic vein. Am J Cardiol. 1989; 63:483–488. PMID: 2644801.

24. von Herbay A, Frieling T, Häussinger D. Association between duplex Doppler sonographic flow pattern in right hepatic vein and various liver diseases. J Clin Ultrasound. 2001; 29:25–30. PMID: 11180181.

25. Zhang-An , Himura Y, Kumada T, Hayashida W, Ishikawa N, Noda M, Kohno F, Kambayashi M, Kawai C. The characteristics of hepatic venous flow velocity pattern in patients with pulmonary hypertension by pulsed Doppler echocardiography. Jpn Circ J. 1992; 56:317–324. PMID: 1578603.

26. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003; 16:777–802. PMID: 12835667.

Fig. 1
Pulsed-wave Doppler waveform of the hepatic vein in a normal dog, with simultaneous electrocardiography recording. Four wave components are noted in the normal tetraphasic waveform. A, atrial systolic wave; S, systolic wave; V, ventricular systolic wave; D, diastolic wave.
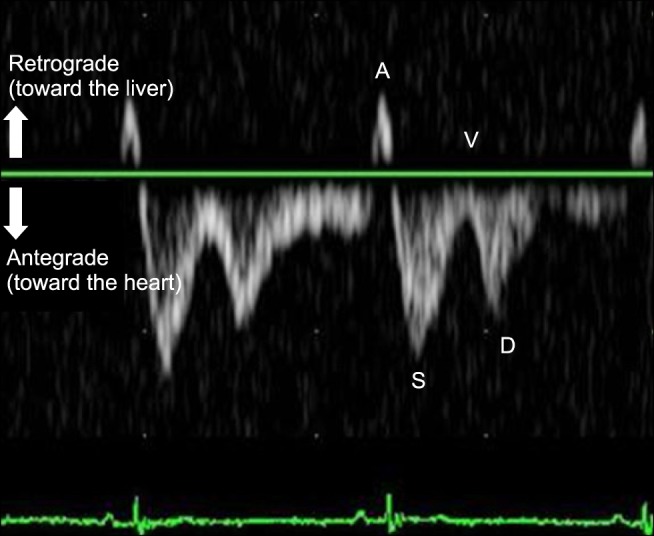
Fig. 2
B-mode with a color Doppler image of the quadrate and right medial hepatic veins. The Doppler gate is placed within the quadrate hepatic vein with 1.5 mm of sample volume. Note the location of the two blood vessels and the gall bladder (GB), and the parallel relationship between the Doppler beam and the quadrate hepatic vein (QHV). RMHV, right medial hepatic vein.
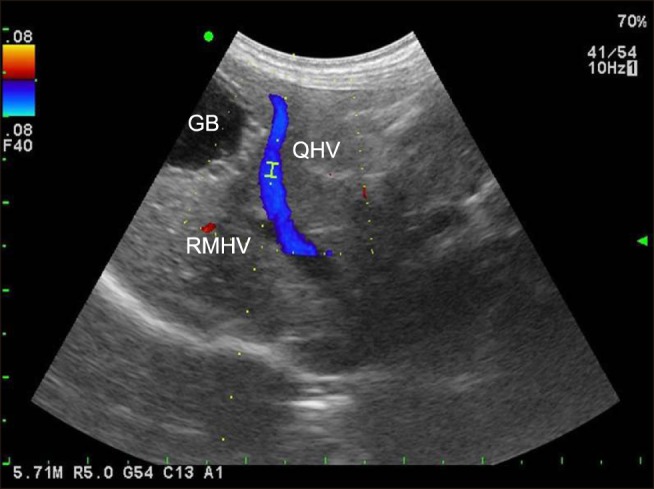
Fig. 3
Three types of hepatic venous flow patterns in dogs with tricuspid regurgitation (TR). (A) Type 1 (normal systolic dominance) waveform with mild TR. (B and C) Type 2 (systolic blunting) waveform with moderate TR and the ventricular systolic (V)-waves of various sizes. (D) Type 3 (systolic reversed) waveform with severe TR. Note that the atrial systolic (A)-, systolic (S)-, and V-waves are fused and show a large retrograde pulse. D, diastolic wave.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download