Abstract
In quadrupeds, the three-dimensional orientation of the heart with respect to the thorax is fundamentally different from that in humans. In this study, we assessed the best surgical approach to the tricuspid valve in sheep. Firstly, different surgical access sites to the tricuspid valve were tested in sheep cadavers, the anatomy was analyzed, and the optimal surgical approach to the tricuspid valve was determined. Secondly - along with cardiopulmonary bypass and cardioplegic arrest -the chosen approach was tested in six adult sheep in vivo. Anatomical analyses revealed that a left thoracotomy provided optimal access to the aorta and left heart. However, visualization of the right heart was significantly impaired. In contrast, a right thoracotomy provided good access to the right heart, but the ascending aorta was difficult to approach. Therefore, in the in vivo studies, arterial cannulation was performed through a carotid (n = 4) or femoral (n = 2) artery. In conclusion, a right-sided thoracotomy allows good visualization of all components of the tricuspid valve complex in sheep, but not of the ascending aorta. Consequently, peripheral vessels are preferred for arterial cannulation. This work may stimulate the investigation of pathomechanisms and/or novel treatment options for tricuspid valve pathologies.
Large animal experiments with quadrupeds are frequently performed to investigate pathomechanisms of different cardiac pathologies (e.g., mitral regurgitation or heart failure) and/or to test novel treatment options (e.g., novel heart valve designs or mechanical support systems) [578]. The three-dimensional orientation of the heart with respect to the thorax is, however, fundamentally different in quadrupeds than in humans. As a consequence, surgical approaches to access individual structures of the heart in quadrupeds are not intuitive for a cardiac surgeon trained to operate on human patients. While there has been extensive research on the left heart and mitral valve in quadrupeds, including descriptions of surgical strategies [124], studies investigating the right heart and the tricuspid valve have been reported less frequently [9].
One possible reason for the relatively small number of experimental studies focusing on mechanisms leading to tricuspid valve disease may be uncertainty about the optimal surgical access to approach the tricuspid valve in quadrupeds. We set out to develop and describe a surgical strategy that allows good visualization of all components of the tricuspid valve complex in sheep.
Sheep cadavers were analyzed to determine relevant surgical structures (cardiac anatomy and peripheral vessels) from two different surgical access sites (right and left thoracotomy). Afterward, 6 female sheep (mean weight, 48 ± 6 kg) were operated via right thoracotomy, and the strategy to surgically approach the tricuspid valve complex was subsequently refined (see Results). By performing cardiopulmonary bypass and cardioplegic arrest, radiopaque markers (commercially available hollow silver balls; diameter, 2 mm; weight, 0.003 g) were attached to all components of the tricuspid valve complex.
Sheep were premedicated with ketamine (10 mg/kg intramuscularly; Zoetis, Germany) and midazolame (0.1 mg/kg intramuscularly; Rotexmedica, Germany), intubated, and mechanically ventilated with inhalational isoflurane (1.0%–2.5%; AbbVie, Germany). Analgesia was reached by intravenous application of 0.002 mg/kg fentanyl (Rotexmedica) per hour. Sheep were relaxed with pancuronium (0.1 mg/kg intravenously; Inresa, Germany).
All animal experiments adhered to relevant regulations and were approved (No. 22-2684-04-02-032/11) by the Thuringia Animal Welfare Committee, Thuringia, Germany.
Anatomical analyses revealed that a left thoracotomy provided optimal access to the aorta, but visualization of both caval veins and the tricuspid valve complex was significantly impaired. In contrast, a right thoracotomy provided good access to both caval veins and permitted straightforward visualization of the tricuspid valve complex. As a consequence, all in vivo studies were performed via a right thoracotomy with the sheep lying in a left lateral position (panel A in Fig. 1). Panels B-D in Fig. 1 show the surgical situs after cutting down the left femoral artery (panel B in Fig. 1B), the right common carotid artery (panel D in Fig. 1), and the opening of the thorax (panel C in Fig. 1). The surgical strategies used to approach the tricuspid valve, including potential surgical pitfalls, are described below in more detail.
In order to surgically visualize the tricuspid valve complex, different positions from the surgeon with respect to the sheep were tested. Positioning the surgeon on the front/abdominal side of the sheep allowed straightforward opening of the thorax and placement of all cannulas, whereas a surgeon positioned on the back side of the sheep allowed best access to the tricuspid valve. Panels A and B in Fig. 2 schematically show the surgeon standing either on the abdominal/front side (panel A in Fig. 2) or the back side (panel B in Fig. 2) of the sheep.
The lower border of the scapula was a good anatomical landmark for the beginning of the skin incision. The end of the skin incision was the sternal border. Fat and muscles were then dissected down to the ribcage. In all animals, the fourth intercostal space provided the best access to the tricuspid valve complex. During the opening of that intercostal space, the right thoracic artery and vein were visualized and ligated.
Panel C in Fig. 1 shows the situs after insertion of the rib retractor. The phrenic nerve runs along the upper border of the pericardium. After insertion of the rib retractor, the pericardium was opened and the heart suspended in a pericardial cradle (panel C in Fig. 2). At that juncture, the right atrium and ventricle, as well as both caval veins, are clearly visible and easy to access. In this study, implantation of radiopaque markers on the valve leaflets required cardioplegic arrest. Therefore, the ascending aorta and brachiocephalic trunk had to be clearly exposed. Panel C in Fig. 1 demonstrates that the aorta cannot be seen via a right thoracotomy without additional exposure. In order to visualize the aorta, the right atrial appendage and pulmonary artery need to be retracted. Panel D in Fig. 2 shows the situs after manual retractions of the right atrial appendage, pulmonary atrium, and pulmonary artery. The ascending aorta has been dissected from the pulmonary artery and is encircled by vessel loops. As compared to humans, where the ascending aorta is in general about 4–5 cm long, the ascending aorta in sheep is significantly shorter (2–3 cm) due to early branching of the brachiocephalic trunk (similar to cattle, sheep have a common brachiocephalic trunk from which all supra-aortic vessels [right and left carotid and subclavian arteries] branch). It is therefore technically impossible to place the aortic and cardioplegic cannulae in the ascending aorta at the same time. Furthermore, the aortic arch and descending aorta were difficult to visualize from this surgical access. As a consequence, we preferred to use the peripheral vessels (carotid and femoral arteries) for placement of the aortic cannula (see ‘Anatomy and surgical access to the peripheral vessels’).
Panel E in Fig. 2 illustrates the situs after cannulation of both caval veins and insertion of the cardioplegic cannula. After aortic cross clamping and cardioplegia, the surgeon switched to the back side of the sheep (panel B in Fig. 2), and the right atrium was opened. Panel F in Fig. 2 shows the tricuspid valve annulus, leaflets, chords, and papillary muscles after implantation of the radiopaque markers. The next paragraph describes the strategy to induce and maintain cardiac arrest.
Cardioplegic arrest was induced by using warm, potassium-enriched blood cardioplegia in an antegrade fashion. The cardioplegic cannula was placed in the ascending aorta (Fig. 2E). At this time, it is of great importance to visualize the brachiocephalic trunk as it can either be misidentified as the descending aorta or may be overlooked (i.e., the cross clamp may either be placed on the trunk or the descending aorta erroneously). Both complications occurred in one of the six sheep in this study, resulting in insufficient cardiac arrest. Furthermore, unlike humans, sheep commonly have a vein that drains blood from the upper part of the body into the coronary sinus (equivalent to a left persistent caval vein in humans). This vein is, however, located on the left side of the heart and cannot be encircled or occluded from a right-sided surgical access. A retrograde delivery of cardioplegia, therefore, proved to be infeasible via a right thoracotomy. Other vessels in sheep may also significantly differ from those in humans. This is especially true for the femoral vessels as described in the following paragraph.
Preparation of peripheral vessels may be needed for invasive pressure monitoring and/or for peripheral placement of arterial or venous cannulae for the heart-lung machine. The locations of the left femoral and right carotid arteries are displayed in panels B and D in Fig. 1, respectively. The inguinal canal in sheep is not as easy to palpate as that in humans. Furthermore, it is of note that the positions of the femoral vessels change significantly depending on the degree of flexion/extension of the leg; moreover, the femoral vessels may be located underneath thick muscle bundles (panel B in Fig. 1). Due to these anatomical complexities, in four of the six sheep the carotid artery was used for arterial cannula placement and the femoral artery was punctured for arterial pressure monitoring. Panel D in Fig. 1 shows the locations of the right jugular vein and carotid artery after surgical exposure. In sheep of this size (approximately 50 kg) the jugular vein is 2 to 3 cm in diameter, is easily palpable, and lies directly underneath the skin. The skin should be incised medially (towards the trachea) from the jugular vein to avoid damage to the vessel. The right common carotid artery lies deeper and is medial to the jugular vein (panel D in Fig. 1). In one of the six sheep, implantation of venous cannulae through the femoral and carotid veins was tested; however, the femoral vein diameter was smaller than the cannula and the approach had to be abandoned.
Table 1 shows a stepwise protocol that allows surgical access to the tricuspid valve through a right thoracotomy with total cardiopulmonary bypass and cardioplegic arrest. Table 2 summarizes potential pitfalls that should be considered when undertaking tricuspid valve surgery using cardioplegic arrest via a right thoracotomy in sheep.
We describe a safe, reproducible strategy that allows good surgical exposure of the tricuspid valve complex in sheep by performing right thoracotomy with cardiopulmonary bypass and cardioplegic arrest. Tricuspid valve surgery in quadrupeds is associated with several pitfalls, which are mostly due to anatomic differences from human beings. The description of our applied surgical strategy and its potential pitfalls may help to simplify experimental tricuspid valve surgery in quadrupeds. Few reports have described surgical strategies to approach the tricuspid valve in quadrupeds. Gilbert et al. [3] described experimental reconstruction of the tricuspid valve with autologous fascia lata, but no full cardiopulmonary bypass was used. Kinney et al. [6] described a trans-sternal bilateral thoracotomy in a canine model with acute, reversible tricuspid insufficiency. That access route appears to be relatively invasive and might impair the recovery of the animal in cases in which chronic studies are to be performed. Walter et al. [9] described the creation of a tricuspid regurgitation model in which a right thoracotomy was used for surgical access to the tricuspid annulus; however, the procedure was described only briefly and a cardiopulmonary bypass was not needed. In our study, since surgical placement of radiopaque markers required cardiopulmonary bypass and cardioplegic arrest, a strategy to induce and maintain cardioplegia was needed. We have described our surgical strategy in great detail and found it to be safe, straightforward, and reproducible with good visualization of all components of the tricuspid valve complex.
With regard to the arterial cannulation site, we observed that, if a right thoracotomy is used, the ascending aorta lies behind the pulmonary artery and therefore is difficult to access. As a consequence, we recommend using peripheral vessels (carotid or femoral artery) for arterial cannulae placement. However, two things should be considered if arterial cannulae for a heart-lung machine are to be placed in peripheral vessels. First, the size of the vessels may be too small to allow placement of an arterial cannula of a sufficient size. In such cases, placement of a second arterial cannula in a different vessel may be an alternative. Second, if the size of the vessel is small, placement of an arterial cannula may result in temporary occlusion of the vessel with the risk of local clot formation and, eventually, thromboembolism after cannula removal. In order to avoid a cannulation-related thromboembolism, we recommend the placement of a vessel clamp distal from the cannulation site prior to the removal of the cannula. After the cannula has been taken out, the clot formation can be easily removed and the vessel reopened.
The in vivo experiments reported herein were performed as acute investigations, i.e., the sheep were not recovered. Therefore, it cannot be determined whether the developed surgical strategy is feasible in a chronic experimental setting. Furthermore, anatomy in other quadrupeds may differ from those in sheep.
In conclusion, tricuspid valve surgery via a right-sided thoracotomy and using a cardiopulmonary bypass allows good visualization of all components of the tricuspid valve complex in sheep. However, access to the ascending aorta is impaired when using that surgical approach. As a consequence, arterial cannulation of peripheral vessels such as the carotid artery is preferred. The results of this study may stimulate further experimental research on the tricuspid valve in quadrupeds and, ultimately, help to identify more fully the pathomechanisms leading to tricuspid valve disease.
Figures and Tables
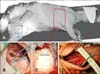 | Fig. 1Sheep in a left lateral position with neck and left groin prepared for puncture/cannulation of the peripheral vessels (A). The location of the left femoral and right carotid artery is displayed in B and D, respectively. (B) The position of the femoral vessels changes significantly depending on the degree of flexion/extension of the leg, and the femoral vessels may be located underneath thick muscle bundles. (C) The surgical situs after insertion of the rib retractor. The phrenic nerve runs along the upper border of the pericardium. (D) The right common carotid artery after surgical exposure. The catheters in the upper right corner are lying in the jugular vein. |
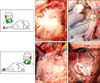 | Fig. 2Schematics of a sheep in a left lateral position and the surgeon standing either on the front (A) or back (B) side of the sheep. The first part of the surgery (until opening of the right atrium) was performed from the front side (C-E). The pericardium was opened and the heart suspended in a pericardial cradle (C). Right atrium (RA) and ventricle (RV) as well as both caval veins (vena cava inferior [VCI] and vena cava superior [VCS], respectively) and coronary sinus (CS) are clearly visible and easy to access. In this study, implantation of radiopaque markers on the valve leaflets required cardioplegic arrest. Therefore, aorta and brachiocephalic trunk had to be clearly exposed. Note that aorta and pulmonary artery (PA) cannot be seen via a right thoracotomy without additional exposure (C). (D) The situs after manual retraction of the RA. The ascending aorta (AAo) has been dissected from the PA and is encircled by vessel loops. The AAo is significantly shorter in sheep (2–3 cm) than in humans (4–5 cm) due to an early branching of the brachiocephalic trunk (BCT). The blue line indicates the distal end. (E) The situs after cannulation of both caval veins and insertion of the cardioplegic cannula (arrow). After aortic cross clamping the surgeon switched locations to the back side of the sheep (F). (F) Tricuspid valve annulus (TA; yellow line), tricuspid septal leaflet (TSL), and the heads of the tricuspid septal papillary muscle (TSPM) after implantation of radiopaque markers. |
Acknowledgments
We thank Ms. Juliane Adolph, DVM, and Ms. Katrin Hornung, MA, for their expert help with conducting the experiments and Ms. Stefanie Kratzke, BSc, for help with the photographs. This animal experiment was funded by research grant J 26, Interdisziplinäres Zentrum für Klinische Forschung (IZKF), Friedrich Schiller University, Jena, Germany.
References
1. Bothe W, Chang PA, Swanson JC, Itoh A, Arata K, Ingels NB, Miller DC. Releasable annuloplasty ring insertion—a novel experimental implantation model. Eur J Cardiothorac Surg. 2009; 36:830–832.

2. Bothe W, Kuhl E, Kvitting JPE, Rausch MK, Göktepe S, Swanson JC, Farahmandnia S, Ingels NB Jr, Miller DC. Rigid, complete annuloplasty rings increase anterior mitral leaflet strains in the normal beating ovine heart. Circulation. 2011; 124:11 Suppl. S81–S96.

3. Gilbert JW Jr, Mansour K, Sanders S, Gravanis MB. Experimental reconstruction of the tricuspid valve with autologous fascia lata. Arch Surg. 1968; 97:149–153.

4. Glasson JR, Komeda M, Daughters GT, Bolger AF, Karlsson MO, Foppiano LE, Hayase M, Oesterle SN, Ingels NB Jr, Miller DC. Early systolic mitral leaflet “loitering” during acute ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 1998; 116:193–205.

5. Hendry PJ, Ascah KJ, Rajagopalan K, Calvin JE. Does septal position affect right ventricular function during left ventricular assist in an experimental porcine model? Circulation. 1994; 90(5 Pt 2):II353–II358.
6. Kinney TE, Olinger GN, Sagar KB, Boerboom LE. Acute, reversible tricuspid insufficiency: creation in canine model. Am J Physiol. 1991; 260:H638–H641.

7. Lai DT, Tibayan FA, Myrmel T, Timek TA, Dagum P, Daughters GT, Liang D, Ingels NB Jr, Miller DC. Mechanistic insights into posterior mitral leaflet inter-scallop malcoaptation during acute ischemic mitral regurgitation. Circulation. 2002; 106:12 Suppl 1. I40–I45.

8. Timek TA, Dagum P, Lai DT, Liang D, Daughters GT, Tibayan F, Ingels NB Jr, Miller DC. Tachycardia-induced cardiomyopathy in the ovine heart: mitral annular dynamic three-dimensional geometry. J Thorac Cardiovasc Surg. 2003; 125:315–324.

9. Walter EMD, Vasilyev NV, Sill B, Padala M, Jimenez J, Yoganathan AP, Hetzer R, del Nido PJ. Creation of a tricuspid valve regurgitation model from tricuspid annular dilatation using the cardioport video-assisted imaging system. J Heart Valve Dis. 2011; 20:184–188.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download