Abstract
One-year-old male Persian cat presented with multiple fractures and no known traumatic history. Marked decrease of bone radiopacity and thin cortices of all long bones were identified on radiography. Tentative diagnosis was osteogenesis imperfecta, a congenital disorder characterized by fragile bone. To determine bone mineral density (BMD), quantitative computed tomography (QCT) was performed. The QCT results revealed a mean trabecular BMD of vertebral bodies of 149.9 ± 86.5 mg/cm3. After bisphosphonate therapy, BMD of the same site increased significantly (218.5 ± 117.1 mg/cm3, p < 0.05). QCT was a useful diagnostic tool to diagnose osteopenia and quantify response to medical treatment.
Osteogenesis imperfecta (OI) is a genetic bone disorder of collagen type I production, involving connective tissues and bones. It is characterized by brittle bones that fracture easily with minimal or absent trauma [2]. In veterinary medicine, OI has been reported in cattle, sheep, tiger, dogs, and cats [1671213]. Diagnosis of OI is based on revealed multiple fractures, fibroblast analysis, and DNA study in human medicine [10]. The diagnosis of OI in veterinary medicine is based on postmortem histopathologic examination and DNA study [13]. However, DNA examination may not be readily available in veterinary clinics. Therefore, definite diagnosis of OI is complicated in small animals. In human medicine, measurement of bone mineral density (BMD) is a routine examination to diagnose osteopenia or osteoporosis. In patients with OI, BMD assessment is routinely used in screening or identifying effect of medical treatment [9]. In veterinary medicine, evaluation of BMD and body composition by using dual energy X-ray absorptiometry (DEXA) and quantitative computed tomography (QCT) have been studied previously [48]. In this report, BMD was measured to diagnose osteopenia and to evaluate medical response after bisphosphonate therapy in an OI-suspected cat.
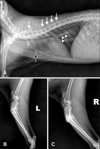
A one-year-old neutered male cat was referred to our institution with a history of recurrent multiple fractures in long bones over a period of 6 months. The cat was bright and alert, and the cat had been regularly fed a commercial well-balanced diet (Indoor Intense Hairball 34 dry cat food; Royal Canin, France). Body weight of the cat was 2.5 kg, rectal temperature was 38.2℃, heart rate was 160 beats per min, and respiratory rate was 30 times per min. The cat was unable to walk or bear weight in all limbs. Neurologic deficit was not identified, and a specific problem was not identified in dental examination. Complete blood count and serum chemistry including calcium (9.2 mg/dL; reference range, 8.0–11.8 mg/dL) and phosphorus (6.8 mg/dL; reference range, 3.5–8.5 mg/dL), revealed no abnormal values. Survey radiography showed multiple fractures including olecranon of right ulna, bilateral tibia, bilateral fibula, bilateral ileum, and bilateral ischium. In addition, the left side 5, 8, 10, 11, and 12th ribs and the right side 6, 8, 10, 11, and 12th ribs had fractures. The third sternum and spinous processes of the 2, 5, 6, 7, and 8th thoracic vertebrae also showed fractures. Some fractures were in the healing process with callus formation, but most fractures remained in non-union or malunion condition. In addition, general bone densities of vertebrae and long bones appeared decreased and cortices of these bones were relatively thin (Fig. 1). To rule out hyperparathyroidism and rickets, additional examinations were performed, which revealed normal intact parathormone (2.2 pmol/L; reference range, 0–4 pmol/L), 25-hydroxy vitamin D (91.1 nmol/L; reference range, 65–170 nmol/L), and ionized calcium (1.36 mmol/L; reference range, 1.2–1.4 mmol/L) values.
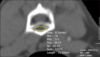
To quantify bone density, QCT was performed. Prior to examination, bone density curve slope was acquired by using calcium hydroxyapatite bone phantom (CIRS Phantom; Computerized Imaging Reference Systems, USA). The mean computed tomography (CT) numbers for three regions containing calcium were regressed against known calcium concentrations (50, 100, and 150 mg/cm3 of calcium hydroxyapatite) to determine the slope of the calibration curve for each image (Fig. 2).
The cat was scanned in dorsal recumbency within the CT scanner (HiSpeed CT/e; GE Healthcare, USA) under general anesthesia. The scanning protocols were as follows: spiral scan, 120 kV, 100 mA, 1.5 mm thickness, and 1.3 pitch. All CT images were analyzed and measured in a constant window (window width 1000; window level 200). To measure trabecular BMD (tBMD), Hounsfield units (HU) in the region of interest (ROI) were determined for all lumbar vertebral bodies (Fig. 3). For HU measurement of vertebral bodies, an elliptical ROI of the trabecular bone without beam hardening artifacts was drawn. The HU value for each ROI was converted to equivalent tBMD (mg/cm3) by applying the linear regression result from the bone density phantom. The obtained mean tBMD of the vertebral bodies was 149.9 ± 86.5 mg/cm3.
Based on the QCT result, OI was tentatively diagnosed, and treatment was initiated with vitamin C (ascorbic acid, 75 mg/kg, per orally q24h; Koreaeundan, Korea) and bisphosphonate (Pamiron, 1 mg/kg, IV per 4 weeks; Bcworld Pharm, Korea) for 1 month. After 4 weeks of treatment, follow-up QCT was performed to evaluate the therapeutic response. Post-treatment mean BMD was 218.5 ± 117.1 mg/cm3. Although tBMD increased after a month of treatment, additional fractures were observed in long bones on follow-up CT images. Since clinical improvement had not been obtained, further examination and therapy was not undertaken, as per owner's request.
In this case, mean tBMD at presentation was 149.9 ± 86.5 mg/cm3. It has been reported that the normal range of tBMD obtained via QCT in lumbar vertebral bodies of cats is 257–315 mg/cm3, whereas BMD obtained via DEXA is 583 ± 4 mg/cm3 [48]. Considering this case's much lower tBMD than that in normal cats, a tentative diagnosis of osteopenia was made. Upon laboratory examination, hyperparathyroidism and rickets were excluded. Secondary nutritional hyperparathyroidism was not completely excluded by the laboratory results, but there was no evidence of malnutrition or gastrointestinal problems. Therefore, secondary nutritional hyperparathyroidism was ruled out.
In a previous report on tBMD of normal cats, tBMD was measured in lumbar vertebrae and the iliac body [4]. However, bilateral iliac body fractures were observed in this patient; therefore, long bone tBMD could not be determined accurately. Moreover, bone biopsy was not attempted due to the risk of additional fracture.
OI is a disease of the skeletal system that is uncommonly observed in small animal practice. It is caused by mutations in the COL1A1 and COL1A2 genes that encode type I procollagen. Type I collagen is an important component of skin, tendons, ligaments, cartilage and bone, and a lack of type I collagen leads to bone fragility and pathological fractures [2]. There is no cure for OI, but bisphosphonate and vitamin C administration have been applied as therapeutics [25]. In human medicine, intravenous bisphosphonate administration has been reported [5]. In children with OI, BMD and clinical signs in serial examination improve markedly with bisphosphonate therapy [9]. In the present case, clinical improvement was not shown following bisphosphonate and vitamin C administration, but there was a significant increase in tBMD identified via QCT. Regardless, the post-treatment tBMD failed to reach a normal level, resulting in additional pathologic fractures. The patient's general outcome did not improve after a month of treatment. A definitive diagnosis of OI can be made by culturing fibroblasts from skin biopsy specimens, which is a complex diagnostic method and hardly practical in small animal practice [10]. Using a bone densitometric method in small animals is a relatively simpler and less invasive method to evaluate OI and other diseases exhibiting osteopenia.
In determining BMD, variable modalities such as DEXA and QCT are available, and previous studies on BMD in cats have used various modalities to measure BMD [34811]. However, there are only a few reports about measuring BMD in cats with secondary osteopenia. Therefore, tBMD measurement by using QCT may be acceptable in osteopenia cases such as OI. By undertaking QCT examination, quantification of bone density is possible, and the results are more accurate than those obtained via radiography or plain CT. Moreover, if applied post-treatment, it can be useful in evaluating the response to medical treatment.
Figures and Tables
Fig. 1
Thoracic radiography image (A), and images of left hindlimb (B) and right hindlimb (C). In (A), multiple fractures of spinal process of thoracic vertebra (white arrows) are visible. Multiple rib fractures (arrowheads) and a 3rd sternum fracture (black arrow) are also visible. The tibia and fibula are fractured bilaterally and inappropriate bone healing was suspected.
References
1. Arthur DG, Thompson KG, Swarbrick P. Lethal osteogenesis imperfecta and skin fragility in newborn New Zealand Romney lambs. N Z Vet J. 1992; 40:112–116.

2. Burnei G, Vlad C, Georgescu I, Gavriliu TS, Dan D. Osteogenesis imperfecta: diagnosis and treatment. J Am Acad Orthop Surg. 2008; 16:356–366.

3. Cann CE. Quantitative CT for determination of bone mineral density: a review. Radiology. 1988; 166:509–522.

4. Cheon H, Choi W, Lee Y, Lee D, Kim J, Kang JH, Na K, Chang J, Chang D. Assessment of trabecular bone mineral density using quantitative computed tomography in normal cats. J Vet Med Sci. 2012; 74:1461–1467.

5. Dimeglio LA, Ford L, McClintock C, Peacock M. A comparison of oral and intravenous bisphosphonate therapy for children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2005; 18:43–54.

6. Horvarth SA, Francesetti FL, Riveros SV. [Treatment of imperfect osteogenesis in a tiger (Panthera tigris)]. Av Cienc Vet. 1986; 1:49–51. Spanish.
7. Jensen PT, Rasmussen PG, Basse A. Congenital osteogenesis imperfecta in Charollais cattle. Nord Vet Med. 1976; 28:304–308.
8. Lauten SD, Cox NR, Baker GH, Painter DJ, Morrison NE, Baker HJ. Body composition of growing and adult cats as measured by use of dual energy X-ray absorptiometry. Comp Med. 2000; 50:175–183.
9. Plotkin H, Rauch F, Bishop NJ, Montpetit K, Ruck-Gibis J, Travers R, Glorieux FH. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab. 2000; 85:1846–1850.

11. Sartoris DJ, Resnick D. Dual-energy radiographic absorptiometry for bone densitometry: current status and perspective. AJR Am J Roentgenol. 1989; 152:241–246.

12. Scott PP, Mckusick VA, Mckusick AB. The nature of osteogenesis imperfecta in cats. Evidence that the disorder is primarily nutritional, not genetic, and therefore not analogous to the disease in man. J Bone Joint Surg Am. 1963; 45:125–134.




 Citation
Citation Print
Print



 XML Download
XML Download