Abstract
Opinions on ovariohysterectomy (OHE) of bitches vary depending on region and country. In this descriptive, prospective cross-sectional study, uterine tracts and ovaries exhibiting gross pathologic findings (n = 76) were collected post-surgery from a reference population of 3,600 bitches (2.11% incidence) that underwent elective OHE during September to November 2013 and evaluated by histopathology examination. Data were evaluated by using descriptive statistics and chi-squared tests. Bitches were of crossbred background with average age 5 years (range 0.6–8.0 years) and most were nulliparous (69.7%) with no anamnesis of reproductive diseases (81.6%). Frequencies of proestrus, estrus, and diestrus were 42.1%, 6.6%, and 19.7%, respectively. The presence of mammary gland masses (5.3%) significantly correlated with histopathologic findings in ovaries and age of the bitch (p < 0.05). Predominant uterine histopathologies included cystic endometrial hyperplasia, periglandular fibrosis, lymphoplasmocytary endometritis, and adenomyosis (19.7%, 14.5%, 4.0%, and 2.6%, respectively). In ovaries, hyperplasia of rete ovarii, follicular cysts, oophoritis, adenoma of the rete ovarii, cysts of superficial structures, and granulosa cell tumors (10.5%, 10.5%, 7.9%, 4.0%, 2.6%, and 2.6%, respectively) were observed. The results reveal the presence of subclinical pathologies in healthy bitches, suggesting that OHE at an early age is beneficial for prevention of reproductive pathologies.
Endogenous progesterone, an estradiol, exerts physiological effects in the ovary and uterus of bitches and acts as an inductor of proliferation in ovarian and uterine epithelium [35]. Canines are particularly susceptible to the deleterious effects of sex steroids; e.g., prolonged exposure to progesterone and/or estradiol results in ovarian tumor (mainly granulosa cell tumor) and cystic endometrial hyperplasia-pyometra (CEHP) complex [137]. The stimulatory effects of progesterone and estradiol on ovarian and uterine epithelium during consecutive estrus cycles act as risk factors for the establishment of proliferative conditions in these tissues as well as in mammary gland epithelium and stroma [12]. The risks of occurrence of ovarian pathologies and CEHP increase proportionally with bitch age [345], thus raising questions about the optimal age for gonadectomy in dogs. Moreover, nulliparity was associated with an increased risk of pyometra in Finnish dogs [28]. On those bases, ovariohysterectomy (OHE) at an early age has been recommended if the dog is not considered for breeding purposes [1838].
Reproductive pathologies such as pyometra and mammary gland neoplasia can be detected at clinical examination, especially in older bitches. However, several pathologies are not currently diagnosed because of their subclinical nature, as is the case with ovarian neoplasia that is clinically evidenced at advanced ages [578]. Most ovarian and uterine subclinical pathologies evolve to more complicated clinical entities such as malignant mammary neoplasia and CEHP, thereby compromising the dog's life [129]. There are few studies revealing the incidence of uterine pathologies and their relationships to infertility or repeat breeding in dogs [25]. There is an absence of reports on the frequency of ovarian and uterine pathologies in healthy dogs, but there is one report in which almost 50% of dogs showed at least one genital pathology at post-mortem examination [29]. Accordingly, the objective of this study was to describe uterine and/or ovarian histopathologic findings following gross and microscopic examination of a sample of clinically healthy dogs undergoing elective OHE.
The ethics advisory committee of CES University at Medellin, Colombia approved the study (Code No.12, Act No. 2, November 27 2012).
The study was defined as a descriptive, prospective cross-sectional study that included a sample of 76 crossbred bitches, mean age 5.3 years (0.5–8 years), randomly selected from a population of 3600 bitches that had undergone elective OHE. From the 76 bitches, extracted uteri and ovaries exhibiting gross pathology were selected for microscopic evaluation.
Sample size was calculated on the basis of a population of 100,000 dogs in the city of Medellin, Columbia (according to the Quality of Life Survey from Municipality of Medellin, Colombia, 2009). The formula used for calculation of sample size for a descriptive cross-sectional study was [6]: where, n = sample size of the study; z2 = 1.96 for a significance level of α = 0.05, with two tails; p = 4.8%, the estimated proportion of the characteristic of interest (in this case, the proportion of cystic endometrial hyperplasia, the most frequent characteristic); q = p (1 − p); and e2 = Error allowed with respect to proportion. The dog population in the city of Medellin (N) was assumed to be a finite population of 100,000 dogs.
Application of the above formula resulted in a sample size of 76 dogs affected by macroscopic pathologic findings at gross examination, immediately after elective spaying. Individual samples were obtained consecutively over a period of three months (September to November 2013) until completion of sample size (76 bitches).
Bitches included in the study had a crossbred genetic background and were surgically spayed in several neighborhoods of Medellin city. All bitches were attended as part of the annual public-policy program of massive sterilization for natal control of dogs and cats in the Municipality of Medellin. OHE was performed in mobile surgical units by a specialized team of veterinarian surgeons from the School of Veterinary Medicine, University of Antioquia, each one of them with more than 20 years of experience in massive spaying of dogs and cats. For all dogs, informed consent was obtained from each dog's owner. Surgical procedures were performed during the warm-humid season of the year (September to November) when the temperature in the city averages 26℃. The age of the dogs operated upon ranged between 0.5 and 8 years.
Before surgical intervention, each bitch was subjected to hematologic evaluation, and only dogs exhibiting normal hematologic findings underwent the surgical spaying procedure. Bitches were premedicated with acepromazin (0.04 mg/kg intravenous [IV], Acedan; Holliday Scott, Argentine) and ketamine (8–10 mg/kg IV, Ketamine 50; Holliday Scott). Anesthesia was maintained with propofol (2 mg/kg IV, Propofol 1%; Claris Lifesciences, India). A lateral laparotomy was performed in the right paralumbar fossa under general anesthesia. The ovaries and uterus were exposed through a 4 to 6 cm cranial to caudal incision comprising the middle third of the paralumbar fossa. The ovarian pedicles were sutured using a transfixion suture with a 2-0 multifilament, synthetic absorbable suture material (Poliglactina 910; Ethicon, USA). Similarly, the cervical end was sutured by using a transfixion pattern with 2-0 Poliglactina 910. Suturing of the lateral abdominal wall was performed with 2-0 or 3-0 Poliglactina 910, and closure of the skin was completed by using a continuous simple pattern with 2-0 monofilament nylon suture (Dermalon; Ethicon). As part of the massive sterilization program's protocol, all dogs were subjected to post-surgical evaluation until complete recovery after general anesthesia. Suture points were retired 10 days after surgery.
After surgical removal of ovaries and the uterine tract, researchers performed gross examination to detect pathologic findings in uteri and ovaries. The uterine tract was excised with a scalpel in order to look for the presence of any kind of secretion and/or macroscopic abnormalities. Gross uterine findings included abnormal color, neoplasia, and abnormal fluids that were in turn classified as serous, mucous, mucopurulent, purulent, hemorrhagic, or hemorrhagic/purulent. When an abnormality was observed, the bitch was defined as a case and clinical evaluations of the vulva and mammary glands were performed in order to detect the presence of abnormal secretions and/or mammary gland masses, respectively. In addition, the uterine tract and ovaries were embedded in 10% formaldehyde and processed with hematoxylin and eosin staining. In addition, a vaginal swab was taken with two smears obtained for estrus cycle classification based on vaginal cytology. Cytology smears were stained by using Diff-Quik stain.
After surgical extraction of uterus and ovaries, researchers undertook gross examination of the uterus, uterine mucosa, and ovaries. Uterine horns were cut with a number 15 scalpel blade and the uterine lumen completely evaluated for macroscopic pathologies. The gross findings included congestion, hemorrhage, tumoral masses, malformations, fibrosis, and abnormal luminal uterine secretions resembling purulent or mucopurulent material. Samples were taken of each site exhibiting gross pathology and were fixed in 10% buffered formalin solution until processing for microscopic evaluation.
From every dog, whose uterine tract and/or ovary exhibited a gross pathologic finding, a single swab was taken with the dog under general anesthesia for subsequent vaginal cytology examination. Subsequently, smears were fixed and transported to the laboratory until processing by Diff-Quik staining. The percentages of parabasal cells, intermediate cells, and superficial cells were calculated twice after counting 100 cells using a 40× objective. The presence of other cells such as erythrocytes, leukocytes, and bacteria was also recorded and their presence classified into four categories: none, low, middle, and abundant. Based on examination of one vaginal smear, cytological results were classified into four categories according to the method described by Groppetti et al. [13]: anestrus, if parabasal cells and intermediate cells predominated; proestrus, if parabasal cells and 10 to 70% of superficial cells predominated; estrus, if more than 85% of superficial cells predominated with or without erythrocytes; and diestrus, if intermediate and parabasal cells predominated with abundant neutrophils and no evidence of bacterial contamination.
From the 76 uterine tracts in the study sample, 216 pieces of affected tissue were taken from uteri and/or ovaries and were processed for microscopic evaluation. All samples were embedded in paraffin, sectioned at 5 mm, and stained with hematoxylin and eosin. Histopathologic evaluation was performed by using a Leica DMLB microscope (Meyer Instruments, USA). Microscopic fields containing pathological findings were photomicrographed by using a digital Leica EC3 camera (Leica Microsystems, Switzerland) with a resolution of 3 megapixels. All evaluations were performed by the same veterinarian pathologist at the Laboratory of Animal Pathology, School of Veterinary Medicine, University of Antioquia, Medellin, Colombia.
For comparison purposes, bitches with gross uterine pathologies were classified into four age groups: Females < 1 year-old (6.6% of dogs), females between 1 and 3 years-old (44.7% of dogs), females between 4 and 6 years-old (31.6% of dogs), and females > 7 years-old (17.1% of dogs) (Table 1).
All data were tabulated in Excel and processed by using StatgraphicsPlus. Qualitative variables are expressed as relative and/or absolute frequencies. Quantitative variables are presented as means ± standard deviation. The significance level (p) was established as 0.05. The chi-squared test was used to compare age groups. Uterine and ovarian findings were analyzed by using contingency tables in a 2 × 2 model. The presence or absence of microscopic findings was contrasted with age, parity, and the presence of mammary neoplasia and uterine lesions as observed by researchers. In addition, data from the survey related to previous reproductive diseases of an infectious nature (evidenced by vaginal discharge), previous administration of anti-conceptive treatment, and previous pseudo pregnancy were also analyzed.
All bitches included in the study had a crossbred genetic background, and most dogs (76.3%) were between 1 and 7 years old (Table 1). At the time of spaying, the dogs were in proestrus (n = 32, 42.1%), estrus (n = 5, 6.6%), diestrus (n = 15, 19.7%), and anestrus (n = 3, 3.9%). There was no report of previous whelping in 69.7% of the cases, whereas 18.4%, 3.9%, and 1.3% of cases had undergone 1, 2, and 4 whelping episodes, respectively (Table 2). Absence of previous reproductive disease was indicated in 81.6% of the cases, whereas infectious diseases (no specific disease data reported by owners), infertility, or abortion were reported in 14.5%, 2.6%, and 1.3% of cases, respectively (Table 3). Nine of the 14 cases (64.3%) with previous reproductive disease received medical treatment; 1 dog receiving hormonal treatment and the remaining 8 dogs receiving antibiotics. Previous cases of pseudo pregnancy were reported by owners in 34.2% of the cases. Sixty-four of the 76 cases did not receive hormonal treatment for estrus suppression, whereas the remaining 12 cases (15.8%) received at least one such treatment.
Only 4 of the 76 cases (5.3%) had mammary masses at gross evaluation, but a statistically significant relationship was detected between bitch age and mammary mass presence (p = 0.0148). Uterine fluid consistency was mucous, mucopurulent, and hemorrhagic in 40.8%, 14.5% and 2.6% of cases, respectively. No uterine secretion was observed in 40.8% of cases. The most frequent gross findings were neoplasia, presence of abnormal uterine content, and abnormal color of mucosae in 36.8%, 59.2%, and 17.1% of cases, respectively. No gross findings were observed in vulva or vestibule.
In 34 of the 76 cases, no histopathological findings were recorded. In the remaining 42 cases, cortical hemorrhage, hyperplasia of rete ovarii (Fig. 1), follicular cysts (panel A in Fig. 2), oophoritis, adenoma of the rete ovarii, cysts of superficial structures, and granulosa cell tumor (Panel B in Fig. 2) were observed in 19.7%, 10.5%, 10.5%, 7.9%, 3.9%, 2.6%, and 2.6% of cases, respectively. These findings were not significantly different between the left (panel A in Fig. 3) and right (panel B in Fig. 3) ovaries (Table 4).
Microscopic lesions were detected in 48.7% of cases and included cystic endometrial hyperplasia (panels A and B in Fig. 4), periglandular fibrosis (panels A and B in Fig. 4), mixed endometritis, lymphoplasmocytary endometritis, adenomyosis (Fig. 5), and mesonephric duct cyst (Fig. 6) in 19.7%, 14.5%, 4.0%, 4.0%, 2.6%, and 2.6% of cases, respectively. Less frequently, adenocarcinoma, mononuclear endometritis, degenerative fibrosis of endometrial glands, and endometrial hemorrhage (1.3% each) were observed (Table 4).
In this study, the frequencies of histopathological findings in uteri, ovaries, and uterine tracts obtained from clinically healthy dogs in a population undergoing elective OHE were examined. Mammary neoplasia, abnormal uterine fluid, and uterine neoplasia were observed in 5.3%, 17.0%, and 36.8% of cases, respectively. Similar incidences were reported by Ortega-Pacheco et al. [29], in a population of stray dogs in which 45% of the animals exhibited at least one genital pathology at post-mortem examination. In our sample of uterine tracts obtained from live dogs, high levels of histopathological abnormalities were found in uteri and ovaries (59.0% and 48.7%, respectively). The most frequent histopathological findings in ovaries were hyperplasia of the rete ovarica, follicular cysts, and oophoritis, whereas, in uteri, the commonest were endometrial hyperplasia and periglandular fibrosis. Interestingly, these findings were observed in dogs assumed to be clinically healthy at the time they were selected for elective OHE.
Based on the observed incidence of gross uterine findings, our sample inclusion criteria resulted in almost half of the cases exhibiting some form of uterine (59.0%) or ovarian (48.7%) pathology at microscopic evaluation. Other authors have reported a relationship between nongonadectomy and a high incidence of pathologies in uteri [123], ovaries [430], and mammary glands [112636], suggesting that OHE should be always considered if the dog is not owned for reproduction purposes [12].
In the present study, cortical hemorrhage was present in 15 and 14 cases in left and right ovary, respectively. It is a common finding after OHE due to manipulation of the ovaries during the surgical procedure. Ovarian neoplasia is usually a post-mortem finding and is uncommon in bitches, and it can be observed in spayed dogs because of neoplastic transformation of ovarian remnants [1420]. Our data provide evidence of the occurrence of ovarian neoplasia (comprised of adenoma of the rete ovarii and granulosa cell tumor) in 6.6% of clinically healthy bitches.
Ovarian tumors in bitches are the result of neoplastic transformation of epithelial, germinal, or stromal cells [24]. Ovarian tumors of epithelial origin are more frequent than tumors of germinal [30] or stromal [162024] cell origin. Epithelial-origin tumors include epithelial cell tumors, tumors of rete ovary, and of subepithelial structures such as papillary adenoma, papillary carcinoma, cyst adenoma, cyst adenocarcinoma, and non-differentiated carcinoma [101124]. The most important tumor of stromal cell origin is the granulosa cell tumor [1230], which was detected in 3.9% of cases in the present study. The sertoli pattern of granulosa tumor observed in the present study was also reported by our group in a case report on a bitch with a previous history of repeat breeding [12]. Another notable finding in the present study was the presence of follicular cysts in eight of the 76 cases. Anovulatory cysts are reported as incidental findings in old bitches in which they originate because of a lack of the preovulatory LH surge and, thus, should be differentiated from cysts of superficial epithelial structures or ovarian neoplasia [17].
Our findings on the presence of both follicle cyst and cyst of superficial epithelial structures are in agreement with the report by Ortega-Pacheco et al. [29] in which the authors reported a relationship between cyst incidence and bitch age. Similarly, Patnaik and Greenlee [30] reported a significant relationship between the frequency of mammary tumors and bitch age. Because most of the dogs in our study were under 6 years old, our results provided no strong evidence of an association between bitch age and incidences of uterine and/or ovarian pathologies. Oophoritis was one of the most frequent findings in ovaries in the present study, but we were unable to locate previous reports indicating the frequency of oophoritis in healthy or infertile bitches; however, there was one case report in which the authors observed oophoritis concomitant with abnormal corpus luteum function in a bitch [27].
Uterine pathologies, particularly neoplasia, are not diagnosed on a routine basis in dogs [22021], and some clinical entities are first observed at an advanced stage of the disease, as is the case with CEHP [834]. Our histopathologic lesion results provide evidence of the occurrence of reproductive pathologies at an early age in bitches. Because proliferative changes in uterus and ovaries could be a consequence of continuing exposure to steroid hormones, the uterine epithelium may be prone to develop neoplasia at early life [20], as was observed in the present study. In a recent report by Patsikas et al. [31], ultrasound examination of 9 dogs revealed the presence of masses with solid images, which were observed to be three cases of leiomyoma, two cases of adenocarcinomas, and one case of leiomyoma. In that same report, dogs exhibiting cysts and cystic masses at ultrasound evaluation of the uterus, those cysts and masses were identified as fibroleiomyoma and leiomyoma at microscopic evaluation [31]. Balka et al. [2] reported an endometrial adenoacanthoma in an 8-year-old bitch after elective OHE. Pires et al. [33] described the immunophenotype of an endometrial adenocarcinoma found in the uterine body of a 10-year-old female Boxer. Endometrial polypoid adenomatosis has been reported in a bitch affected by granulosa cell tumor [39]. Finally, Pena et al. [32] reported the concomitant presence of endometrial adenocarcinoma with mucometra in a 6-year-old Alaska Malamute bitch.
In addition to the abovementioned case reports, the only report on the frequency of uterine neoplasia in bitches was that by Ortega-Pacheco et al. [29]. In our study, adenomyosis and adenocarcinoma occurrences were the most frequent findings in uteri of clinically healthy dogs (< 3%). On the other hand, the most frequent non-neoplastic uterine pathologies were cystic endometrial hyperplasia and periglandular fibrosis (19.7% and 14.5%, respectively). Uterine hyperplasic proliferations include adenomyosis, cystic endometrial hyperplasia, uterine polyps [19], and miscellaneous uterine cysts such as lymphatic cysts, persistent ducts, remnants of embryo structures related to sexual differentiation, mesonephric duct remnants, cysts of serous inclusions, and endometrial cysts derived of dilated uterine glands [24]. Our results were obtained from clinically healthy bitches and are similar to those reported by Mir et al. [25] in a study of bitches affected by unexplained infertility. Those authors reported that fibrosis with degeneration of endometrial glands and endometritis, cystic endometrial hyperplasia, and mixed lesions were the most frequent findings [25].
Cystic endometrial hyperplasia is considered one of the most important proliferative disorders of the canine endometrium and is closely related to pyometra [1915]. This complex disease is usually diagnosed within 4 weeks to 4 months after estrus and it has a variable prevalence depending mainly on the spaying policies of each region or country, thus suggesting influences associated with socioeconomic factors [2229]. In Sweden, a country where spaying is not widely accepted, pyometra was diagnosed in 25% of cases in 10-year-old nongonadectomized bitches [38]. Notably, cystic endometrial hyperplasia is a well-recognized risk factor for pyometra in nongonadectomized bitches [13437].
In addition to the effect of sex steroids on the proliferative pathologies of reproductive epithelia, it is also important to consider their effect on proliferative disorders of the mammary gland. Most cases of neoplastic transformation in nongonadectomized bitches are neoplasia of the mammary gland (incidences of up to 3.4%) [30]. The effect of sex steroids on mammary gland epithelium increases the risk of mammary neoplasia. Indeed, one of the most important consequences of gonadectomy is a significant reduction in the incidence of mammary gland neoplasia. Most cases of mammary gland neoplasia in bitches are both malignant (50.9%) and metastatic [112030]. The authors of the 2008 Animal Tumor Registry of Genoa, Italy reported an increased incidence of mammary tumors in bitches ranging from 3 years old (mean 10.6, range 7.1–15.1 per 100,000 bitches) to 11 years old (mean 561.2, range 507.5–619.0 per 100,000 bitches), and it was found that mammary gland tumor was the most frequent neoplastic transformation in bitches [23]. A retrospective study performed in the Veterinary Pathology Service at University of Antioquia (n = 232 samples) comprised a 30 year (1968–1998) retrospective evaluation, and the results showed that carcinoma was the most frequent mammary gland neoplasia (58.6%) [10]. In an observational study by Gómez et al. [11] a carcinoma incidence of 81% was observed in nongonadectomized bitches (average age 10.6 years) and lung metastasis was observed in 17% of the cases.
The risk of developing mammary gland neoplasia is low if practicing spaying before the first or second estrus cycle (0.5% and 0.8%, respectively). This risk increases to 26% if spaying occurs after the third estrus cycle [35]. In our study, only 5.3% of cases exhibited mammary gland neoplasia. This value could be lower than expected because our diagnoses of mammary gland masses was based on clinical examination with no subsequent confirmation by biopsy and histopathologic evaluation. In our study, a significant relationship was observed between the presence of mammary masses and age (p < 0.5), as has been previously reported [36].
In summary, clinically healthy bitches undergoing elective OHE exhibited several pre-existing reproductive pathologies, many of them located at the ovaries (55.2%) and uterus (48.7%) as evidenced by microscopic evaluation. Moreover, the most common finding at gross examination was normal uterine fluid (40.8% of cases) and mucopurulent material (14.5% of cases). It was also observed that almost half of the dogs between 0.5 and 8 years old exhibited some type of ovarian and/or uterine pathology. The most frequent microscopic findings in ovaries were hyperplasia of the rete ovarica (10.5%) and follicular cysts (10.5%), whereas the most frequent microscopic findings in uteri were endometrial hyperplasia (19.7%) and periglandular fibrosis (14.5%). In our study, a significant correlation was found between the presence of mammary masses at clinical exam and bitch age. Most bitches were in the active phase of the estrus cycle, suggesting that estrus-related behavior may be one of the factors dog owners take into account before spaying their dogs.
Figures and Tables
Fig. 1
Proliferative disorders found in ovaries. (A) Epithelial proliferation of the rete ovarii (asterisks). (B) Stratification of the rete ovarii showing epithelial proliferation in a different ovary from that of panel A. H&E stain. 200× (A), 400× (B).
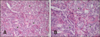
Fig. 2
Follicle cysts. (A) Microscopic aspect of the cyst wall (cw), ovary cortex (c), ovary medulla (m). (B) Granulosa cell tumor exhibiting a sertoli pattern; connective tissue (ct). H&E stain. 40× (A), 200× (B).
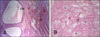
Fig. 3
Frequency of occurrence of microscopic lesions in the left (A) and right (B) ovaries at elective ovariohysterectomy in clinically healthy bitches with uteri containing gross pathologic findings.

Fig. 4
Cystic endometrial hyperplasia. (A and B) Dilated cystic glands (asterisk) from two different cases. Endometrium (em). Endometrium fibrosis. (C and D) severe periglandular fibrosis (asterisks) with abundant active fibroblasts (arrow). Endometrium (em) and endometrial glands (g). H&E stain. 40× (A, B, and D), 200× (C).
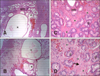
Fig. 5
(A) Adenomyosis showing cystic glands (arrow); myometrium (m), vascular layer (v) and hyperplasic endometrial glands with cysts (asterisks). (B) Adenomyosis, in which groups of endometrial glands appears embedded into the myometrium (arrow). H&E stain. 100× (A), 40× (B).

Fig. 6
(A) Mesonephric duct cyst showing the endometrium (em), myometrium (m) and cyst wall (arrows). (B) Cysts of superficial epithelium containing cystic structures of several sizes are seen in the ovarian cortex (asterisks). Cumulusoocyte-complex (coc) are present. H&E stain. 400× (A), 100× (B).

Table 1
Frequency distribution by age at elective ovariohysterectomy in clinically healthy bitches with uteri containing gross pathologic findings

Table 2
Frequency distribution for parity status at elective ovariohysterectomy in clinically healthy bitches with uteri containing gross pathologic findings

Acknowledgments
Authors wish to thank CES University and Universidad de Antioquia (Medellin, Colombia) for financial support for the research activities of the INCA-CES Research Group and the Centauro Research Group, respectively. Special thanks to “Sustainability Strategy for Excellence Ressearch Groups 2013–2014, University of Antioquia”.
References
1. Arora N, Sandford J, Browning GF, Sandy JR, Wright PJ. A model for cystic endometrial hyperplasia/pyometra complex in the bitch. Theriogenology. 2006; 66:1530–1536.

2. Balka G, Szabó L, Jakab C. First report of an endometrial adenoacanthoma in a dog. Acta Vet Hung. 2011; 59:225–236.

3. Bhatti SF, Rao NA, Okkens AC, Mol JA, Duchateau L, Ducatelle R, van den Ingh TS, Tshamala M, Van Ham LM, Coryn M, Rijnberk A, Kooistra HS. Role of progestin-induced mammary-derived growth hormone in the pathogenesis of cystic endometrial hyperplasia in the bitch. Domest Anim Endocrinol. 2007; 33:294–312.

4. Bostedt H, Jung C, Wehrend A, Boryzcko Z. [Clinical and endocrinological findings of bitches with ovarian cyst syndrome]. Schweiz Arch Tierheilkd. 2013; 155:543–550. German.
5. Brønden LB, Nielsen SS, Toft N, Kristensen AT. Data from the Danish veterinary cancer registry on the occurrence and distribution of neoplasms in dogs in Denmark. Vet Rec. 2010; 166:586–590.

6. Colimon KM. [Fundamentals of Epidemiology]. 3a ed. Medellín: Corporación para Investigaciones Biológicas;2010. p. 551. Spanish.
7. Concannon PW, Spraker TR, Casey HW, Hansel W. Gross and histopathologic effects of medroxyprogesterone acetate and progesterone on the mammary glands of adult beagle bitches. Fertil Steril. 1981; 36:373–387.

8. De Bosscher H, Ducatelle R, Tshamala M, Coryn M. Changes in sex hormone receptors during administration of progesterone to prevent estrus in the bitch. Theriogenology. 2002; 58:1209–1217.

9. De Bosschere H, Ducatelle R, Vermeirsch H, Van Den Broeck W, Coryn M. Cystic endometrial hyperplasia-pyometra complex in the bitch: should the two entities be disconnected. Theriogenology. 2001; 55:1509–1519.

10. Ferreira de la Cuesta G. [Veterinary Pathology]. lst ed. Medellin: Editorial Universidad de Antioquia;2003. p. 526–530. Spanish.
11. Gómez B, Ramírez M, Maldonado-Estrada J. Presence of lung metastases in bitches affected by malignant mammary neoplasms in Medellin (Colombia). Rev MVZ Cordoba. 2012; 17:2983–2990.
12. González-Domínguez MS, Fernández LB, Saldarriaga S, Aranzazu-Taborda D, Maldonado-Estrada JG. [Infertility in a bitch with history of recurrent reproductive failure associated with granulosa cell tumor]. Rev Colomb Cienc Pecu. 2005; 18:258–268. Spanish.
13. Groppetti D, Pecile A, Arrighi S, Di Giancamillo A, Cremonesi F. Endometrial cytology and computerized morphometric analysis of epithelial nuclei: a useful tool for reproductive diagnosis in the bitch. Theriogenology. 2010; 73:927–941.

14. Günzel-Apel AR, Buschhaus J, Urhausen C, Masal C, Wolf K, Meyer-Lindenberg A, Piechotta M, Beyerbach M, Schoon HA. [Clinical signs, diagnostic approach and therapy for the so-called ovarian remnant syndrome in the bitch]. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2012; 40:35–42. German.
15. Hagman R, Lagerstedt AS, Hedhammar Å, Egenvall A. A breed-matched case-control study of potential risk factors for canine pyometra. Theriogenology. 2011; 75:1251–1257.

16. Ichimura R, Shibutani M, Mizukami S, Suzuki T, Shimada Y, Mitsumori K. A case report of an uncommon sex-cord stromal tumor consisted of luteal and sertoli cells in a spayed bitch. J Vet Med Sci. 2010; 72:229–234.

17. Knauf Y, Bostedt H, Failing K, Knauf S, Wehrend A. Gross pathology and endocrinology of ovarian cysts in bitches. Reprod Domest Anim. 2014; 49:463–468.

18. Kustritz MV. Determining the optimal age for gonadectomy of dogs and cats. J Am Vet Med Assoc. 2007; 231:1665–1675.

19. Marino G, Barna A, Rizzo S, Zanghì A, Catone G. Endometrial polyps in the bitch: a retrospective study of 21 cases. J Comp Pathol. 2013; 149:410–416.

21. McGavin MD, Zachary JF. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis: Elsevier;2012. p. 1079–1092.
22. McKay SA, Farnworth MJ, Waran NK. Current attitudes toward, and incidence of, sterilization of cats and dogs by caregivers (owners) in Auckland, New Zealand. J Appl Anim Welf Sci. 2009; 12:331–344.

23. Merlo DF, Rossi L, Pellegrino C, Ceppi M, Cardellino U, Capurro C, Ratto A, Sambucco PL, Sestito V, Tanara G, Bocchini V. Cancer incidence in pet dogs: findings of the Animal Tumor Registry of Genoa, Italy. J Vet Intern Med. 2008; 22:976–984.

24. Meuten DJ, editor. Tumors in Domestic Animals. 4th ed. Ames: Iowa State Press;2003. p. 176–179.
25. Mir F, Fontaine E, Albaric O, Greer M, Vannier F, Schlafer DH, Fontbonne A. Findings in uterine biopsies obtained by laparotomy from bitches with unexplained infertility or pregnancy loss: an observational study. Theriogenology. 2013; 79:312–322.

26. Misdorp W. Progestagens and mammary tumours in dogs and cats. Acta Endocrinol (Copenh). 1991; 125:Suppl 1. 27–31.
27. Nickel RF, Okkens AC, van der Gaag I, van Haaften B. Oophoritis in a dog with abnormal corpus luteum function. Vet Rec. 1991; 128:333–334.

28. Niskanen M, Thrusfield MV. Associations between age, parity, hormonal therapy and breed, and pyometra in Finnish dogs. Vet Rec. 1998; 143:493–498.

29. Ortega-Pacheco A, Gutiérrez-Blanco E, Jiménez-Coello M. Common lesions in the female reproductive tract of dogs and cats. Vet Clin North Am Small Anim Pract. 2012; 42:547–559.

30. Patnaik AK, Greenlee PG. Canine ovarian neoplasms: a clinicopathologic study of 71 cases, including histology of 12 granulosa cell tumors. Vet Pathol. 1987; 24:509–514.

31. Patsikas M, Papazoglou LG, Jakovljevic S, Papaioannou NG, Papadopoulou PL, Soultani CB, Chryssogonidis IA, Kouskouras KA, Tziris NE, Charitanti AA. Radiographic and ultrasonographic findings of uterine neoplasms in nine dogs. J Am Anim Hosp Assoc. 2014; 50:330–337.

32. Pena FJ, Gines JA, Duque J, Vieitez V, Martinez-Pérez R, Madejón L, Nuñez Martinez I, Moran JM, Fernández-García S. Endometrial adenocarcinoma and mucometra in a 6-year-old Alaska Malamute dog. Reprod Domest Anim. 2006; 41:189–190.

33. Pires MA, Seixas F, Palmeira C, Payan-Carreira R. Histopathologic and immunohistochemical exam in one case of canine endometrial adenocarcinoma. Reprod Domest Anim. 2010; 45:545–549.

34. Pretzer SD. Clinical presentation of canine pyometra and mucometra: a review. Theriogenology. 2008; 70:359–363.

35. Schneider R, Dorn CR, Taylor DON. Factors influencing canine mammary cancer development and postsurgical survival. J Natl Cancer Inst. 1969; 43:1249–1261.
36. Sleeckx N, de Rooster H, Veldhuis Kroeze EJB, Van Ginneken C, Van Brantegem L. Canine mammary tumors, an overview. Reprod Domest Anim. 2011; 46:1112–1131.




 Citation
Citation Print
Print


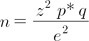


 XML Download
XML Download