Introduction
Atrioventricular valve disease is the most common cardiovascular disease in small-breed dogs, accounting for more than 70% of reported heart disease cases [
10]. The mitral valve is more susceptible than the tricuspid valve to disease and readily undergoes myxomatous degeneration, resulting in myxomatous mitral valve degeneration (MMVD) [
4]. It has been reported that Cavalier King Charles Spaniels, Miniature Poodles, Miniature Schnauzers, Chihuahuas, Pomeranians, Fox Terriers, Cocker Spaniels, and Pekingese breeds tend to be particularly prone to MMVD [
10]. The progression of MMVD leads to congestive heart failure and finally might induce dyspnea, syncope, cyanosis, and even death. In addition, other changes, such as hypertension, pulmonary edema (PE), and arrhythmia can occur [
10]. Diagnosis of MMVD requires a thorough physical examination including auscultation, and, for confirmation, echocardiography should be carried out [
13]. During echocardiography, mitral regurgitation can be imaged by using color-flow Doppler imaging [
1]. Although many medications (
e.g., diuretics, pimobendan, and ACE inhibitors) are available to relieve the symptoms of MMVD [
61517], they are only effective for symptom relief. Thus, expectation of the affected animal's lifespan based on the clinical symptoms during treatment is important in order when providing prognostic information about the dog to its owners. However, many symptoms are possible, and which parameter is an important factor in patient prognosis is unclear.
MMVD is particularly evident in the smallest dog breeds [
11]; however, few studies have evaluated factors affecting the survival time of dogs with MMVD [
2]. The aim of this retrospective study was to describe the signalment and clinical symptoms of 168 small-breed dogs diagnosed with MMVD based on a routine examination and echocardiographic results and to retrospectively review the prognostic variables for and survival time of small-breed dogs with MMVD.
Materials and Methods
Study population
The medical records of small-breed dogs (< 15 kg) examined at the Seoul National University Veterinary Medical Teaching Hospital (SNU VMTH) from 2010 to 2013 were reviewed. One hundred and sixty-eight dogs diagnosed with MMVD during that four-year period were eligible for inclusion in the study. All of the dogs underwent a general clinical examination and a cardiology consultation. During the routine clinical examination, cardiac auscultation and general physical examination were performed to investigate whether the clinical symptoms (i.e., cough and dyspnea) were caused by a cardiovascular disorder, and echocardiography was used to confirm the diagnosis.
Inclusion criteria
Small-breed dogs, weighing less than 15 kg, were included in this study. Physical, chest radiograph, and echocardiographic examinations were performed for all dogs. Some dogs with concurrent disease such as chronic kidney disease, liver failure or hormonal disease (e.g., Cushing's disease, diabetes mellitus, or hypothyroidism) were included only if the condition was well managed with medicinal therapy.
The numbers of dogs showing clinical signs such as cough, cyanosis, dyspnea, exercise intolerance, and syncope were determined based on the chief complaints of owners and the results of physical examination at first visit. If that resting respiratory rate was ≥ 60 breaths/min and was accompanied by shortness of breath, the dog categorized as having dyspnea. One or a combination of echocardiographic features, such as color Doppler identification of any degree of mitral valve regurgitation, two-dimensional (2D) detection of mitral valve prolapse, any degree of mitral valve leaflet thickening or both, were required for inclusion in the study. Dogs were classified as International Small Animal Cardiac Health Council (ISACHC) class I if they did not have present or past clinical signs of congestive heart failure or radiographic evidence of cardiac enlargement. Dogs were classified as ISACHC class II if they had mild to moderate clinical signs at rest or with mild exercise and heart enlargement on thoracic radiographs. Dogs with obvious clinical signs were classified as ISACHC class III.
Exclusion criteria
Dogs with congenital heart disease, bacterial endocarditis, dilated cardiomyopathy, or heartworm infections were excluded because these conditions may have a direct or indirect influence on mitral valve function. Animals with other medical problems that could significantly affect their survival were also excluded (e.g., dogs that had sepsis, neoplasia or immune-mediated hemolytic anemia). Any dogs with clinical evidence of respiratory disease such as the presence of moderate to marked bronchial, interstitial, or alveolar pulmonary patterns were excluded.
Study design
All data, such as signalments (sex, breed, age, body weight, and body condition score [BCS]), clinical symptoms at presentation, progression of symptoms after initial diagnosis, general physical examination (auscultation, arrhythmia, and systolic blood pressure [SBP]), electrocardiogram results, concurrent heart problems, vertebral heart score (VHS), echocardiographic results, and medications being administered, in addition to information on the survival of each dog, were collected retrospectively. Signalments, physical examination results, clinical symptoms, and treatment history were obtained from each case record. The BCS was assessed by using a nine-point body condition scoring system. Heart murmur was graded into 4 scales. The SBP was measured by performing Doppler sphygmomanometry at the first visit to the SNU VMTH. An electrocardiogram was obtained with the animal in a right lateral recumbent position, and the result was interpreted and confirmed by veterinary interns and residents in the Department of Veterinary Internal Medicine. Concurrent cardiovascular diseases, such as tricuspid valve insufficiency (TVI) and PE, were diagnosed via radiography. When tracheal collapse (TC) was suspected, its presence was confirmed by using fluoroscopy.
As part of the cardiac examination, thoracic radiographs were taken left-laterally and ventrodorsally, and the VHS was measured. All dogs underwent complete echocardiographic examination, including transthoracic 2-dimensional, M-mode, spectral, and color-flow Doppler, by three experienced echocardiographers. Experienced echocardiographers in the Department of Veterinary Medical Imaging at SNU VMTH performed the echocardiography with an ultrasound system (ProSound Alpha7; Hitachi, Japan) equipped with a 3–8 MHz phase array sector transducer (Hitachi). A lead II electrocardiogram was simultaneously displayed on the ultrasound monitor for timing purposes. All echocardiographic values were measured in same manner and according to the recommendations of the Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine by using standard methods [
1418]. Measurements of the M-mode left ventricular parameters were obtained from 2D guided M-mode echocardiography in the right parasternal short-axis view at the level of the papillary muscles [
12]. End diastolic and end systolic volumes (EDV and ESV, respectively) of the left ventricle were calculated by using the Teichholz method [
16]. The left ventricular ejection fraction (EF) was calculated by using EF = (EDV – ESV)/EDV × 100. Fractional shortening (FS) was calculated from the left ventricular end diastolic (LVDd) and systolic (LVDs) dimensions by using FS = (LVDd – LVDs)/LVDd × 100. Both EF and FS were determined on the short axis of the M-mode. Peak velocity of tricuspid regurgitation (TR) was obtained under color Doppler guidance. Systolic pulmonary pressure was diagnosed if the peak TR velocity was > 2.8 m/sec.
Assessment of the left atrial (LA) and aortic root (Ao) dimensions was performed from the right parasternal short-axis view at the heart base, and the ratio between LA and Ao was calculated (LA/Ao) [
8]. All measurements were averaged from data during three cardiac cycles. All results were confirmed by a cardiologist of the Department of Veterinary Internal Medicine. Dogs with heart disease were classified into ISACHC heart failure (HF) class I, II, or III. All data including echocardiograms with digital recordings were reviewed by experienced investigators.
A telephone interview with the owner(s) of each enrolled dog was used to ascertain the animal's clinical or survival status. First, the owner was asked whether the dog had survived. If the dog had died, the date of death and its clinical status at the time of death were recorded. In cases where the owner could not be contacted, we contacted veterinarians in the local hospital that had overseen the care of the dog and asked about the animal's clinical status or survival.
Statistical analysis
Statistical analysis was performed by using R package, ver. 3.1.1. Numerical variables are reported as mean ± SD values. The effects of 22 clinical, electrocardiogram, echocardiographic, and Doppler variables on survival were evaluated. The clinical variables included sex, arrhythmia, syncope, dyspnea, PE, age > 11 years, weight > 5 kg, heart rate > 140 beats/min, murmur > II, SBP > 140 mmHg, ISACHC HF class, VHS > 10.5, LA/Ao > 1.5, EDV > 30 mL/m2, and ESV > 5 mL/m2.
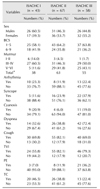
Univariate Cox survival analysis was used to evaluate the hazard ratio of an adverse event. Survival curves, median survival times, and 95% confidence intervals were obtained by applying the Kaplan-Meier method. Survival time was counted from the day of diagnosis of MMVD at the SNU VMTH to either the day of death or the end of the observation period. Differences in survival characteristics of each ISACHC HR class are shown in
Fig. 1. All statistical analyses were conducted and confirmed by a statistician of the Department of Statistics, SNU, Korea.
Discussion
Twenty-two prognostic variables of dogs with MMVD were investigated in this study to allow assessment of their survival-related characteristics. The most significant clinical and echocardiographic factors related to survival duration were dyspnea, PE, and VHS (p < 0.05). The mean survival time of the dogs with dyspnea (327 ± 293 days) was shorter than that of dogs without dyspnea (365 ± 331 days). The presence of PE also increased the dogs with and without PE demonstrated mean survival times of 237 ± 219 days and 379 ± 327 days, respectively.
During HF progress due to MMVD, pulmonary congestion and edema appear. PE can be detected before clinical signs such as dyspnea occur, and it can, directly and indirectly, affect the survival of animals with MMVD. Therefore, early detection and appropriate management of PE could help to prevent dyspnea and deterioration of CHF.
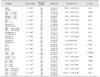
Cardiomegaly has been associated with decreased survival time in dogs with MMVD [
39]. In this study, a VHS over 10.5 vertebrae had a hazard ratio of 2.12 (
Table 5), suggesting that the possibility of death in dogs with a VHS > 10.5 is two-fold higher than that in dogs with a VHS ≤ 10.5. As demonstrated in
Table 3, the mean VHS of dogs in ISACHC class III tended to be higher than that of dogs in ISACHC classes I and II; however, the differences were not significant. When other prognostic variables, such as BCS, age, heart rate, syncope, cyanosis, cough, TVI, TC, arrhythmia, and echocardiographic results (
i.e., EF, FS, PH, LA/Ao, EDV, and ESV) were examined, only age > 11 years, LA/Ao > 1.5, EF > 85, and EDV > 30 mL/m
2, were significantly correlated with overall survival times (
p < 0.1). These prognostic factors are correlated with cardiomegaly and disease progress in mitral valve regurgitation. Although statistically not significant, EDV and ESV were higher in dogs in ISACHC classes II and III, and an EDV > 30 mL/m
2 of EDV tended to be associated with a higher possibility of death (
p = 0.053). The EDV is a preload that stretches the left ventricle of the heart to its greatest dimensions under a variable physiologic demand. When excessive, ventricular volume overload occurs and may result in HF [
10]. In addition, the LA/Ao ratio can be an important prognostic variable. With MMVD progression, the LA dimension increases, and left atrium size is reflective of disease severity. Most of the echocardiographic and radiographic results in this study reflected the appropriate ISACHC classification. Based on echocardiographic results, the higher ISACHC classes were accompanied by increased heart size and increased enlargement of the left atrium. Overall, mitral regurgitation in MMVD can produce a pressure overload on the right heart by increasing pulmonary vascular pressure [
7], and VHS increases during the progression of valvular disease [
5]. Monitoring heart size at initial diagnosis and regularly afterward could assist in assessing the prognosis of dogs with MMVD.
Some of the factors assessed in this study were not significant predictors of survival in small-breed dogs. Body weight, BCS, SBP, arrhythmia, syncope, FS, and end systolic volume were not significantly associated with the risk of death (
p > 0.2). In addition, the sex of the animal with MMVD was not associated with survival duration. An earlier study reported that male dogs are prone to MMVD [
16], but the proportions of each sex in that study population were similar. Moreover, our univariate analysis of sex showed that it did not influence the survival of the dogs with MMVD. In addition, weight did not influence the life expectancy of the dogs. This result is different from that in a study of large-breed dogs [
2]. Although we used a 1 kg difference to stratify the dogs by weight, weight was not a significant factor. In addition, heart murmur intensity was not significantly associated with survival duration of the dogs with MMVD in this study. However, the number of dogs in the low-intensity murmur group (murmur I–II) was too small to allow a statistically powerful comparison with the number of dogs in the higher intensity murmur group (murmur III–VI). Finally, our analysis also showed that an SBP >140 mmHg was not significantly associated with survival. These results differ from those reported by Borgarelli et al. [
2] whose study included large-breed dogs.
As a retrospective study, there are some study limitations. As we focused on small-breed dogs, many dogs with MMVD that were brought to the SNU VMTH had to be excluded. In addition, we were unable to ascertain the survival status of some dogs, and other dogs had to be excluded because they did not meet the inclusion criteria. Moreover, some of the small-breed dogs had undergone only one cardiology consultation, so there was insufficient information for them to be included in the study. Therefore, the results of the current study may not represent a larger population of small-breed dogs. In addition, more than half of the dogs included in the present study were Maltese and Shih Tzu. The results could be biased to those two breeds, thereby limiting the generalizability of the findings to the entire small-breed dog population. Moreover, the predisposition of Maltese, the most popular dog breed in Korea, to MMVD has not been investigated. To determine whether this breed is prone to MMVD, a large percentage of Maltese population in Korea should be studied.
The survival rates and survival duration of dogs that died were analyzed, but the SD of survival duration varied, thereby limiting the statistical analysis. In addition, the drug treatment combinations in the study population were too diverse, and the sample sizes of some groups were too small to allow meaningful statistical analysis. Thus, additional detailed studies with larger study populations are needed; particularly studies of medicinal treatment combinations administered in each of the ISACHC classes.
In conclusion, respiratory distress, directly or indirectly associated with MMVD, is likely to affect survival time in small-breed dogs. The univariate analysis in our study showed that, rather than assessing body weight, BCS, SBP, arrhythmia, syncope, FS, or ESV, clinical assessment of dyspnea, PE, and VHS can be useful in evaluating the survival of dogs with MMVD and can be useful factors when providing prognostic information to owners of small-breed dogs. Among the methods discussed in the current study, measuring the VHS via lateral chest radiographs might be a relatively convenient way of evaluating an animal's prognosis in clinics where echocardiography is not available. In addition, intensive monitoring of VHS after the initial diagnosis should be employed in clinical practice in order to assess the prognosis and modulate the treatment of animals with MMVD.






 Citation
Citation Print
Print



 XML Download
XML Download