Abstract
We previously demonstrated that Bordetella (B.) bronchiseptica antigen (Ag) showed high immunostimulatory effects on mouse bone marrow cells (BMs) while Mycoplasma (M.) hyopneumoniae Ag showed low effects. The focus of this study was to determine if B. bronchiseptica Ag can enhance the M. hyopneumoniae Ag-specific immune response and whether the host's immune system can recognize both Ags. MTT assay results revealed that each or both Ags did not significantly change BM metabolic activity. Flow cytometry analysis using carboxyfluorescein succinimidyl ester showed that B. bronchiseptica Ag can promote the division of BMs. In cytokine and nitric oxide (NO) assays, B. bronchiseptica Ag boosted production of tumor necrosis factor-alpha in M. hyopneumoniae Ag-treated BMs, and combined treatment with both Ags elevated the level of NO in BMs compared to that from treatment of M. hyopneumoniae Ag alone. Immunoglobulin (Ig)G enzyme-linked immunosorbent assay using the sera of Ag-injected mice clearly indicated that B. bronchiseptica Ag can increase the production of M. hyopneumoniae Ag-specific IgG. This study provided information valuable in the development of M. hyopneumoniae vaccines and showed that B. bronchiseptica Ag can be used both as a vaccine adjuvant and as a vaccine Ag.
Mycoplasma (M.) hyopneumoniae is a causative pathogen of swine enzootic pneumonia, leading to significant economic loss in the swine industry [11]. It is widely distributed and occurs in a range of 38%–100% of pigs in swine farms. Although a variety of Mycoplasma vaccines have been developed for disease prevention, their effects are not fully satisfactory [6]. Bordetella (B.) bronchiseptica is another main causative pathogen of the swine respiratory disease atrophic rhinitis [7]. Infection with B. bronchiseptica alone does not seriously damage of respiratory system; however, a combined infection with other pathogens, such as Pasteurella multocida, does result in serious respiratory damage [4], leading to loss of weight and associated economic loss [15]. Thus, a vaccine for B. bronchiseptica was developed [5].
Our previous report demonstrated that M. hyopneumoniae antigen (Ag) has low immunostimulatory activity, although it did induce a T helper 1-type immune response [13]. In contrast, B. bronchiseptica Ag had high immunostimulatory activity and increased cytokine production [14]. These results led us to speculate that both Ags could be combined into a single vaccine if the B. bronchiseptica Ag was able to enhance the production of M. hyopneumoniae Ag-specific immunoglobulin (Ig)G. It has been widely reported that adjuvants, such as alum and MF59, can enhance the immunogenicity of vaccine Ag [13]. Generally, bacteria-derived Ags are not used as a vaccine adjuvant, and their toxic effects have limited their use in the development of vaccines; one example of a bacterial adjuvant is Bacillus Calmette-Guérin (BCG) [12].
In this study, we investigated whether B. bronchiseptica Ag can enhance the M. hyopneumoniae Ag-specific immune responses and if the host's immune system can recognize both Ags.
C57BL/6 and BALB/c mice were purchased from ORIENT BIO (Korea) and were maintained at our animal facility. Female mice of both strains, 7- to 12-weeks-old were used in all experiments. Animal experiments were performed in accordance with the Institutional Guideline for Animal Use and Care of Jeju National University (approval No. 2013-0008). The M. hyopneumoniae and B. bronchiseptica were obtained from CAVAC and KBNP (both in Korea), respectively. Both bacteria were inactivated by treating with formalin and were used as Ags. Following inactivation, they were washed with phosphate-buffered saline (PBS) before use. The amount of protein was measured by performing protein assays (Bio-Rad Laboratories, USA) with the results based on a standard curve derived using bovine serum albumin (Sigma-Aldrich, USA).
Bone marrow cells (BMs) were obtained from the femur and tibia of mice by flushing. To remove red blood cells, BMs were treated with a hypotonic lysis buffer (ammonium chloride-potassium lysis buffer containing 0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA). The cells were filtered through a cell strainer (cut-off: 40 µm) to obtain single cells. The single BMs were then cultured in 5% complete medium (RPMI 1640 medium containing 5% fetal bovine serum, 100 IU/mL penicillin/streptomycin, 2 mM L-glutamine).
For measurement of BM metabolic activity, BMs were cultured in 96-well culture plates at a concentration of 1 × 106 cells/mL (200 µL/well) and treated with the indicated concentrations of Ags. After treatment, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, USA) solution was added at a concentration of 0.5 mg/mL. Subsequently, viable cells generated purple-colored crystals in proportion to their metabolic activity, and 10% (w/v) sodium dodecyl sulfate (Sigma-Aldrich) solution was used to dissolve the crystals. The optical density of the solution was measured at 570 nm by a microplate reader (Multiskan FC; Thermo Fisher Scientific, USA). To investigate whether two Ags affected cellular proliferation, we undertook a proliferation assay using carboxyfluorescein succinimidyl ester (CFSE). The cells were stained 5 µM CFSE and incubated with M. hyopneumoniae Ag and bronchiseptica Ag. After incubation, the cells were analyzed by using FACSCalibur (Beckton Dickinson, USA) and Flowing Software (Finland).
BMs were cultured in 96-well culture plates at a concentration of 1 × 106 cells/mL (200 µL/well) for cytokine or 2 × 106 cells/mL (200 µL/well) for NO assay. Culture supernatants of the Ag-treated cells were harvested and used in both enzyme-linked immunosorbent assay (ELISA) and NO assay. The amount of tumor necrosis factor (TNF)-α in the culture supernatants was measured by using CytoSet kits (Thermo Fisher Scientific) according to the manufacturer's instructions. Optical density was measured at 450 nm by using a microplate reader. The amount of NO in the culture supernatants was measured by using an NO detection kit (iNtRON Biotechnology, Korea) according to the manufacturer's instructions. Optical density was measured at 570 nm by using a microplate reader.
To measure the effects of M. hyopneumoniae Ag and B. bronchiseptica Ag, mice were divided into three groups (each n = 5): PBS, M. hyopneumoniae Ag only, and M. hyopneumoniae Ag + B. bronchiseptica Ag groups. Mice were intraperitoneally injected twice with an interval of 2 weeks between injections. The amounts injected were 10 µg/mouse of M. hyopneumoniae Ag and 5 µg/mouse of B. bronchiseptica Ag. Two weeks after the second injection, mice were sacrificed and used in subsequent experiments.
Mouse blood was collected by cardiac puncture after CO2 euthanasia. After generation of a blood clot, serum was prepared by using a microcentrifuge and serial 5-fold dilution. The detection of Ag-specific serum IgG was carried out by ELISA. Briefly, M. hyopneumoniae Ag or B. bronchiseptica Ag was coated on an ELISA module at a concentration of 5 µg/mL. An assay buffer (Thermo Fisher Scientific) for blocking, as well as goat anti-mouse IgG-horseradish peroxidase and tetramethyl-benzidine solution (both from SouthernBiotech, USA) were sequentially used in the ELISA detection of IgG. Optical density was measured at 450 nm by using a microplate reader.
In order to determine whether B. bronchiseptica Ag or M. hyopneumoniae Ag affects the cell metabolic activity of BMs, we performed MTT assays. After 4 days of treatment with 0 to 10 µg/mL M. hyopneumoniae Ag and 0.4 µg/mL B. bronchiseptica Ag, BM metabolic activity was measured (panel A in Fig. 1). In another treatment set, BMs were treated with 0 to 10 µg/mL B. bronchiseptica Ag and 10 µg/mL M. hyopneumoniae Ag (panel B in Fig. 1). The results showed that the concentration or presence of M. hyopneumoniae Ag and B. bronchiseptica Ag did not markedly alter the cell metabolic activity of BMs. Flow cytometry analysis revealed that B. bronchiseptica Ag marginally enhanced cell size (forward scatter/side scatter), but M. hyopneumoniae Ag did not (panel A in Fig. 2). In addition, B. bronchiseptica Ag increased the percentage of cells with low CFSE fluorescence intensity, indicating the presence of proliferating cells, whereas M. hyopneumoniae Ag did not show such an increase (panel B in Fig. 2).
To investigate the functional immune effects of B. bronchiseptica Ag, TNF-α and NO production was examined. After 3 days of treatment with 0 to 10 µg/mL M. hyopneumoniae Ag with or without 1 µg/mL B. bronchiseptica Ag, the amount of TNF-α was measured. M. hyopneumoniae Ag treatment without B. bronchiseptica Ag did not produce a detectable amount of TNF-α, except at the 10 µg/mL treatment level. The presence of B. bronchiseptica Ag markedly increased the production of TNF-α, whereas M. hyopneumoniae Ag did not significantly alter the production level, except at the 2.5 µg/mL treatment level (Fig. 3). On the other hand, M. hyopneumoniae Ag enhanced NO production in a concentration-dependent manner. M. hyopneumoniae Ag (1.25–10 µg/mL) significantly enhanced NO production in the presence of B. bronchiseptica Ag. In addition, B. bronchiseptica Ag enhanced NO production in 0 to 2.5 µg/mL M. hyopneumoniae Ag treatments to a greater extent than with treatment by M. hyopneumoniae Ag alone (Fig. 4).
To measure the level of M. hyopneumoniae Ag-specific IgG, 5 µg/mL M. hyopneumoniae Ag were coated on ELISA plates. Serum was 5-fold serially diluted and the level of Ag-specific IgG was detected by ELISA (panel A in Fig. 5). No detectable amount of M. hyopneumoniae Ag-specific IgG was measured in the serum of mice injected with PBS only. A significantly higher amount of M. hyopneumoniae Ag-specific IgG was detected in sera of mice injected with M. hyopneumoniae Ag (MH) or injected with M. hyopneumoniae Ag + B. bronchiseptica Ag (MHBB). Furthermore, the level of Ag-specific IgG with the MHBB injection was higher than that from the MH treatment. In addition, the level of B. bronchiseptica Ag-specific IgG was also measured in similar sera samples (panel B in Fig. 5B). A significant amount of B. bronchiseptica Ag-specific IgG was detected in MHBB-injected mice, but not in PBS- and MH-injected mice.
To improve the immunogenicity of Ag, a variety of adjuvants can be used in the process of developing vaccines, and new vaccine adjuvants with high efficiency are in high demand. Bone marrow is the primary organ that produces premature lymphocytes and harbors antibody-producing cells with Ag-specific memory. Immune memory is a critical defense mechanism used to induce more powerful immune responses when the host's immune system is attacked a second time by the same pathogen. Long-lived memory B cells and plasma cells are essential to remove pathogens and act by producing high titers of Ag-specific antibodies [9], a major indicator of vaccine efficacy. For these reasons, we used BMs of mice in this study.
The MTT assay results revealed that treatment of M. hyopneumoniae Ag and/or B. bronchiseptica Ag did not significantly affect the cell metabolic activity of BMs. It is thus likely that these Ags are not significantly cytotoxic to BMs.
TNF-α is a major inflammatory cytokine secreted from macrophages that induces essential immune responses related to vaccine efficacy. Some Ags that induce a low production of TNF-α need adjuvants to induce a high level of immunostimulatory activity. ELISA revealed that M. hyopneumoniae Ag alone produced minimal amount of TNF-α, whereas B. bronchiseptica Ag increased the level when in a combination of M. hyopneumoniae Ag, suggesting that B. bronchiseptica Ag can boost the vaccine efficacy of M. hyopneumoniae Ag.
NO is an intracellular killing factor in cell-mediated-immunity and eliminates pathogenic microorganisms invading host cells [28]. To estimate the intracellular cytotoxic ability of BMs treated by both Ags, the amount of NO was determined in the culture supernatants. NO assay results revealed that the combined treatment of both Ags elevated the level of NO in BMs compared to that after treatment with M. hyopneumoniae Ag alone. Importantly, the effect of B. bronchiseptica Ag was high when it was combined with 0 to 2.5 µg/mL of M. hyopneumoniae Ag. The optimal concentration of M. hyopneumoniae Ag for NO production was 2.5 µg/mL.
CFSE is a cell-permeable, non-fluorescent pro-dye that is cleaved by intracellular esterase in living cells, and a decrease of intracellular CFSE fluorescence intensity indicates cell division [10]. In this study, the CFSE-stained cells were analyzed by flow cytometry, which revealed that the percentage of BMs with a regular cell size decreased following M. hyopneumoniae Ag treatment, but increased with B. bronchiseptica Ag treatment. In addition, the ratio of highly proliferating cells (i.e., showing low fluorescence intensity) showed a similar pattern. Thus, it appears that B. bronchiseptica Ag can promote cell division in BMs.
Plasma cells differentiated from B lymphocytes produce IgG. Bone marrow contains both types of cells and is thus critical in maintaining humoral immunity. In particular, antibody-producing cells with long-term memory are essential for vaccine efficacy. To evaluate the effects of B. bronchiseptica Ag on the M. hyopneumoniae Ag-specific immune response, IgG ELISA was performed using the sera of Ag-injected mice. The level of M. hyopneumoniae Ag-specific IgG was enhanced by B. bronchiseptica Ag. In similar samples, B. bronchiseptica Ag-specific IgG was detected only in B. bronchiseptica Ag + M. hyopneumoniae Ag-injected mice. These results indicate that the IgG ELISA used in the present study was very Ag-specific.
In conclusion, neither M. hyopneumoniae Ag nor B. bronchiseptica Ag had cytotoxic effects on BMs. M. hyopneumoniae Ag slightly decreased the cell size and division, whereas B. bronchiseptica Ag reversed those decreases. M. hyopneumoniae Ag-specific immune response was increased by B. bronchiseptica Ag, which was reflected by an increase in IgG production. This study provides valuable information related to developing vaccines for respiratory diseases, especially M. hyopneumoniae-involved diseases, and shows that B. bronchiseptica Ag can be used as both a vaccine adjuvant and a vaccine Ag. Further study on the development of a new vaccine adjuvant using B. bronchiseptica Ag is needed.
Figures and Tables
 | Fig. 1The effect of Bordetella (B.) bronchiseptica Ag and Mycoplasma (M.) hyopneumoniae antigen (Ag) on the cell metabolic activity of bone marrow cells (BMs). BMs were cultured for 4 days with 0 to 10 µg/mL M. hyopneumoniae Ag and 0.4 µg/mL B. bronchiseptica Ag (A) or with 0 to 10 µg/mL B. bronchiseptica Ag and 10 µg/mL M. hyopneumoniae Ag (B). MTT solution was treated at a concentration of 0.5 mg/mL. The optical density (OD) was measured at 570 nm by using a microplate reader. All values are presented as mean ± SD values from four individual wells. |
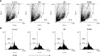 | Fig. 2Bordetella (B.) bronchiseptica antigen (Ag) enhances the proliferation of bone marrow cells (BMs). Carboxyfluorescein succinimidyl ester (CFSE)-stained BMs were treated with 5 µg/mL Mycoplasma (M.) hyopneumoniae Ag and 1 µg/mL B. bronchiseptica Ag for 4 days. Stained cells were analyzed by flow cytometry as described in Materials and Methods. The number in each dot plot or histogram indicates the percentage of normal cell size (A) or the percentage proliferating cells with low fluorescence intensity (B). MH, M. hyopneumoniae Ag-treated BMs; BB, B. bronchiseptica Ag-treated BMs; MHBB, both MH and BB Ags-treated BMs. |
 | Fig. 3Bordetella (B.) bronchiseptica antigen (Ag) promotes the production of tumor necrosis factor alpha (TNF-α) in bone marrow cells (BMs). After 3 days of treatment with 0 to 10 µg/mL Mycoplasma (M.) hyopneumoniae Ag and 1 µg/mL B. bronchiseptica Ag, supernatants of the treated BMs were collected and used for measurement of TNF-α. Optical density was measured at 450 nm by a microplate reader. All values are presented as mean ± SD values from four individual wells. ND, not detectable. *p < 0.05 compared to control (0 µg/mL M. hyopneumoniae Ag), ***p < 0.001 compared between two treatments (with or without B. bronchiseptica Ag). |
 | Fig. 4Mycoplasma (M.) hyopneumoniae antigen (Ag) and Bordetella (B.) bronchiseptica Ag promote the production of nitric oxide (NO) in bone marrow cells (BMs). After 3 days of treatment with 0 to 10 µg/mL M. hyopneumoniae Ag and 0.4 µg/mL B. bronchiseptica Ag, supernatants of the treated BMs were collected and used to measure NO level. Optical density was measured at 570 nm by a microplate reader. All values are presented as mean ± SD values from four individual wells. *p < 0.05, **p < 0.01, and ***p < 0.001, respectively, compared to control (0 µg/mL M. hyopneumoniae Ag). #p < 0.05 and ##p < 0.01, respectively, compared between two treatments (with and without B. bronchiseptica Ag). |
 | Fig. 5Bordetella (B.) bronchiseptica antigen (Ag) treatment increases Mycoplasma (M.) hyopneumoniae Ag-specific immunoglobulin (Ig)G. Mice were injected with phosphate-buffered saline (PBS), M. hyopneumoniae Ag (10 µg/mouse), or M. hyopneumoniae Ag + B. bronchiseptica Ag (5 µg/mouse). After 4 weeks, blood was collected and sera were used for measuring Ag-specific IgG via enzyme-linked immunosorbent assay (ELISA). For this assay, the ELISA module was coated with 5 µg/mL M. hyopneumoniae Ag (A) or B. bronchiseptica Ag (B). The antibody titer was determined at an optical density (OD) of 450 nm. MH, M. hyopneumoniae Ag-treated BMs; BB, B. bronchiseptica Ag-treated BMs; MHBB, both MH and BB Ags-treated BMs. All values are presented as mean ± SD values from four individual wells. *p < 0.05, **p < 0.01, and ***p < 0.001, respectively, compared to the lowest serum concentration (5−10 dilution). #p < 0.05, ##p < 0.01 and ###p < 0.001, respectively, compared between two treatments (MH and MHBB). |
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant Nos. 111065-3 and 109014-03-1-CG000).
References
1. Audibert FM, Lise LD. Adjuvants: current status, clinical perspectives and future prospects. Immunol Today. 1993; 14:281–284.

2. Avron A, Gallily R. Mycoplasma stimulates the production of oxidative radicals by murine peritoneal macrophages. J Leukoc Biol. 1995; 57:264–268.

3. Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O’Hagan DT, De Gregorio E, Seubert A, Wack A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011; 29:1812–1823.

4. Chanter N, Magyar T, Rutter JM. Interactions between Bordetella bronchiseptica and toxigenic Pasteurella multocida in atrophic rhinitis of pigs. Res Vet Sci. 1989; 47:48–53.

5. Chi Y, Lu C, Han JH, Hahn TW. [Efficacy of atropic rhinitis vaccine in pigs]. Korean J Vet Res. 2004; 40:707–717.
6. Haesebrouck F, Pasmans F, Chiers K, Maes D, Ducatelle R, Decostere A. Efficacy of vaccines against bacterial diseases in swine: what can we expect. Vet Microbiol. 2004; 100:255–268.

7. Horiguchi Y. Swine atrophic rhinitis caused by pasteurella multocida toxin and bordetella dermonecrotic toxin. Curr Top Microbiol Immunol. 2012; 361:113–129.

8. MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997; 15:323–350.

9. O'Connor BP, Gleeson MW, Noelle RJ, Erickson LD. The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease. Immunol Rev. 2003; 194:61–76.
10. Parish CR. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol Cell Biol. 1999; 77:499–508.

11. Simionatto S, Marchioro SB, Maes D, Dellagostin OA. Mycoplasma hyopneumoniae: from disease to vaccine development. Vet Microbiol. 2013; 165:234–242.
12. Wangoo A, Brown IN, Marshall BG, Cook HT, Young DB, Shaw RJ. Bacille Calmette-Guérin (BCG)-associated inflammation and fibrosis: modulation by recombinant BCG expressing interferon-gamma (IFN-γ). Clin Exp Immunol. 2000; 119:92–98.

13. Yim SH, Joo HG. [T helper 1-type immunogenicity of Mycoplasma hyopneumoniae antigen on mouse spleen cells]. J Biomed Res. 2013; 14:55–59. Korean.





 Citation
Citation Print
Print


 XML Download
XML Download