Abstract
We evaluated the effects of guanosine 5′-monophosphate (GMP)-chelated calcium and iron (CaFe-GMP) on health and egg quality in layers experimentally infected with Salmonella Gallinarum. In this study, a CaFe-GMP feed additive was added to a commercial layer feed and fed to layers over a four-week period. All were inoculated with Salmonella Gallinarum. Body weight, mortality, clinical symptoms, and poultry production including feed intake, egg production, egg loss, and feed conversion rate were observed, and Salmonella Gallinarum was re-isolated from the liver, spleen, and cecum of the layers. All tested internal organs for the CaFe-GMP additive group exhibited significantly lower re-isolation numbers of Salmonella Gallinarum and less severe pathological changes than those in the control group, indicating that the CaFe-GMP feed supplement induced bacterial clearance and increased resistance to Salmonella Gallinarum. Additionally, due to the inhibitory action of CaFe-GMP on the growth of Salmonella Gallinarum, the CaFe-GMP additive group exhibited better egg production, including a higher laying rate and fewer broken eggs. The results suggest that a 0.16% CaFe-GMP additive may help prevent salmonellosis in the poultry industry.
Fowl typhoid is caused by Salmonella Gallinarum, the transmission route of which is horizontal, i.e., from an infected ovary to the egg and the progeny [3]. The disease can be either acute or chronic and usually results in a high mortality rate (60%) and increased morbidity [229]. Although Salmonella Gallinarum has adapted to its avian host and rarely induces food poisoning in humans, fowl typhoid outbreaks cause severe economic losses worldwide [36].
The worldwide increase in multi-antibiotic resistance in bacteria is a problem caused by major drug resistant nosocomial pathogens. As a result of multiple antibiotic inefficiencies, the mortality rate caused by Salmonella has increased [12]. Recently, several attempts have been made to prevent salmonellosis by using alternatives to antibiotics, such as vaccination, bacteriophages, probiotics, and herbs [101315]. However, these methods have demonstrated limited efficiency. Therefore, the development of efficacious feed additives to prevent poultry salmonellosis is a major research goal [120].
Formulation of balanced diets is fundamental to economical poultry production, and this process depends on knowledge of the nutrient requirements of poultry and the nutritional attributes of various feed sources. Minerals are naturally occurring inorganic solids and dietary nutrients essential in the maintenance of homeostasis for laying hens [22]. In addition, minerals have important roles in pathogen virulence and host antimicrobial resistance [2734]. Calcium enhances intestinal resistance to infectious diseases and protects against Salmonella- and enterotoxigenic Escherichia coli (ETEC)-associated diarrhea [56]. Iron deficiency is associated with lowering resistance to infection, whereas iron supplements reverse this effect [212326].
Recently, organic minerals (chelated minerals), because of their potential for higher bioavailability than inorganic minerals, have been studied by several researchers [19]. Organic minerals include any mineral bound such that it is chelated or bonded to organic molecules, such as an amino acid, thereby forming chemical structures with unique characteristics such as stability and high mineral bioavailability. Several reports have indicated that organic sources of trace minerals have a higher bioavailability than that of inorganic forms [41937]. After absorption, organic minerals may induce physiological effects, which could improve specific metabolic responses, such as the immune response. Many studies have demonstrated the benefits of metal-amino acid chelates on animal metabolism [33].
In this study, we evaluated the effects of the feed additive guanosine 5′-monophosphate (GMP)-chelated calcium and iron (CaFe-GMP), a metal-nucleotide chelate, against salmonellosis in layers.
The CaFe-GMP was provided by Medinutrol (Korea). CaFe-GMP, an organic formulation in which calcium and iron are provided as GMP chelates was used as a supplement to the layers basal diet. A commercial, nutritionally complete, antibiotic-free, general layer feed was used as a basal diet to meet the nutrient requirements for layers in accordance with Nutrient Requirements of Poultry recommendations [22]. The basal diet was formulated to contain a given chemical composition (Table 1).
In our previous study, the potential effect of prebiotics on the growth of Lactobacillus spp. in layers using a feed supplement of 0.16% CaFe-GMP, which contained 83 ppm calcium (Ca) and 55 ppm iron (Fe) (unpublished data) was investigated. In the present experiment, for the CaFe-GMP additive group, CaFe-GMP was completely mixed into the basal diet to form 0.16% CaFe-GMP containing 83 ppm Ca and 55 ppm Fe (Table 1).
Hy-line brown layer chickens (34 weeks old) with no history of salmonellosis were obtained from a local farm. Microbiological testing did not detect Salmonella Gallinarum in the feces of the chickens. All chickens were maintained at the Chonnam National University College of Veterinary Medicine (Chonnam, Korea) in separate air-controlled rooms. Chickens were allowed free access to tap water, and their respective diets were supplied three times per day. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Chonnam National University (approval No. CNU IACUC-YB-2015-59).
After a 7-day acclimation period, the chickens were divided into three groups with six chickens in each group. The groups were as follows: normal group, fed with a normal commercial layer feed without Salmonella Gallinarum inoculation; control group, fed with a normal commercial layer feed but with a Salmonella Gallinarum inoculation; additive group, fed with a normal commercial layer feed supplemented by the addition of CaFe-GMP and with Salmonella Gallinarum inoculation. All chickens were fed their respective diet for 4 weeks, and all but the normal group were inoculated with Salmonella Gallinarum. The experiment was conducted in triplicate.
Salmonella Gallinarum (SG3001) for experimental inoculation was provided by the Animal and Plant Quarantine Agency (Korea). The SG3001 was cultured on MacConkey agar plates overnight. One colony was selected and inoculated in Brain Heart Infusion (Difco Laboratories, USA) broth and incubated at 10 × g for 6 h at 37℃. Viable counts of the broth culture were obtained by using the spread plate method. Inoculated broth was diluted with phosphate buffer saline (PBS; Sigma-Aldrich, USA) to obtain an infective dose of 1 × 108 CFU/mL (CFU, colony-forming unit) (the optimal dose was determined in preliminary experiments). Experimental inoculations were administered orally.
Body weights of chickens were measured at 0 and 5 days post-infection (dpi). The chickens were monitored daily for clinical signs, including anorexia, diarrhea, and depression, among others. Postmortem examinations of all dead and sacrificed chickens were conducted and any pathological changes associated with fowl typhoid were noted. Additionally, the mortality rate in each group during the experimental period was recorded.
Egg production (eggs/chicken/day, %), egg loss (broken and cracked eggs, %), and egg weight (g) were recorded daily. Feed intake (g/chicken/day) was calculated by weighting the initial feed input against the uneaten food over the experimental period. Feed conversion rate (FCR; g feed/g egg) were determined on a daily basis. FCR was expressed as kilograms of feed consumed per kilogram of egg produced. Mortality rate (%) was recorded daily as it occurred.
Re-isolation numbers for Salmonella Gallinarum was determined for liver, spleen, and feces collected from each sacrificed chicken at 5 dpi. In addition, feces were retrieved from sterilized paper that was used to line the chicken's housing unit. For non-selective pre-enrichment processing, 0.1 g of tissue and feces from the cecum of each sample were aseptically collected and added to 10 mL of sterile Buffered Peptone Water (Sigma-Aldrich), homogenized completely, and incubated at 37℃ for 24 h. Then, the homogenate was transferred from 1 mL of the pre-enrichment broth to 10 mL Tetrathionate Broth (TTB; Difco Laboratories) and incubated at 37℃ for 24 h for selective enrichment processing. The TTB was serially diluted 10-fold in PBS, and 50 µL of each dilution was spread onto a Salmonella Shigella agar (Difco Laboratories) plate and incubated at 37℃ for 24 h. The resulting characteristic black-colored colonies were counted and expressed as CFU/0.1 g tissue, but only for those plates with counts of 30 to 300 colonies per plate.
The adverse effects of the Salmonella Gallinarum infection induced in the present study were assessed by examining body weight and clinical symptoms. For each group, body weight change was compared between mean weight at 0 dpi for all chickens in a group to that at 5 dpi in the surviving chickens. The body weights of each group at 0 and 5 dpi are shown in Table 2. The non-inoculated, normal group did not exhibit a change in body weight, whereas the inoculated, control group (1,741.0 g ± 125.0 g) had significantly lower body weight at 5 dpi than at 0 dpi (1,905.7 g ± 155.3 g), a loss of approximately 8.6% (p < 0.07). However, the inoculated, CaFe-GMP additive group exhibited only slightly lower body weight at 5 dpi (1,493.6 g ± 59.4 g) than at 0 dpi (1,598.0 g ± 34.3 g); the change was not significant. The percentage decline in body weight for the CaFe-GMP additive group was less than that of the control group. Clinical signs (anorexia, diarrhea, and depression) were observed in the control group, but the CaFe-GMP additive group showed very few typical clinical signs of Salmonella Gallinarum.
Mortality rate is a major evaluation marker used in treatment toxicity analysis for preclinical studies. Mortality rates in the three trial groups are summarized in Table 3. Mortality was first observed at 4 dpi in the control group, whereas there was no mortality in the CaFe-GMP additive group or the normal group during the experimental period. Although low levels of clinical symptoms of Salmonella Gallinarum were observed in the CaFe-GMP additive group, the group suffered no mortality from the infection.
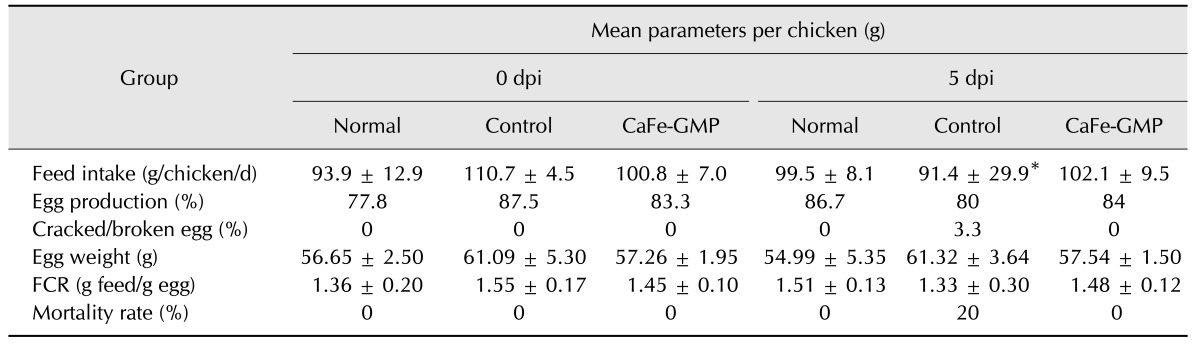
For each group, feed intake was compared at 0 dpi for all chickens and at 5 dpi for all surviving chickens. The mean feed group and (C) CaFe-GMP group. Scale bars = 5 cm. intake for chickens in the normal, CaFe-GMP additive, and control groups at 0 and 5 dpi are shown in Table 3. Compared to 0 dpi, feed intake in the control group decreased significantly by approximately 17.44% at 5 dpi, whereas in the CaFe-GMP additive group it increased slightly (1.2%) at 5 dpi; a non-significant change. The slight increase in feed intake of the CaFe-GMP additive group was less than that in the normal group, although neither increase was significant.
Average egg production rate for the CaFe-GMP additive group was not significantly different from that of the control group before the Salmonella Gallinarum infection period (control group: 87.5%, CaFe-GMP group: 83.3%). After Salmonella Gallinarum infection, the CaFe-GMP additive group maintained a similar laying rate, whereas the laying rate for the control group decreased by 7.5% from that in the uninfected experimental period. The percentage of cracked or broken eggs for the control group after Salmonella Gallinarum infection was 3.3% at 5dpi, whereas no broken eggs occurred in the CaFe-GMP additive group during the experimental period (Table 3).
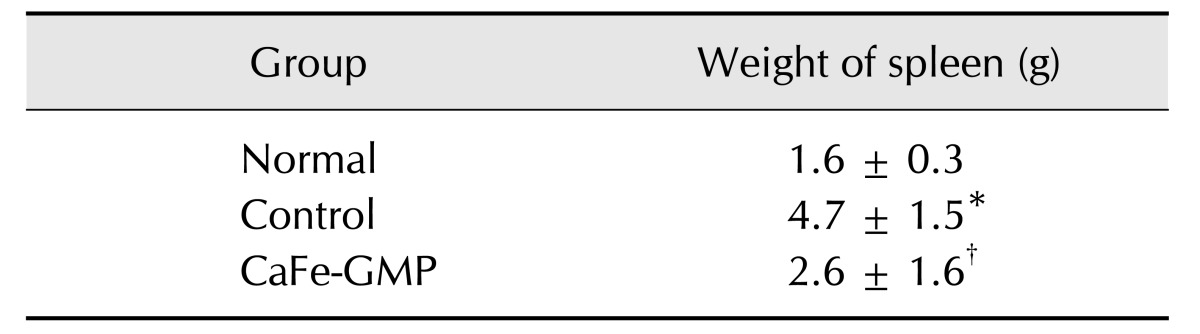
As shown in Table 4, the control group had significantly heavier spleens than those in the normal group. The CaFe-GMP additive group had a spleen weight significantly lower than that in the control group (p < 0.05); moreover, spleen size was similar to that in the normal group (data not shown). In addition, hepatomegaly was reduced in the CaFe-GMP additive group (Fig. 1). Furthermore, necropsy revealed that the CaFe-GMP additive group exhibited milder and less severe gross pathological abnormal changes when compared with those in the control group.
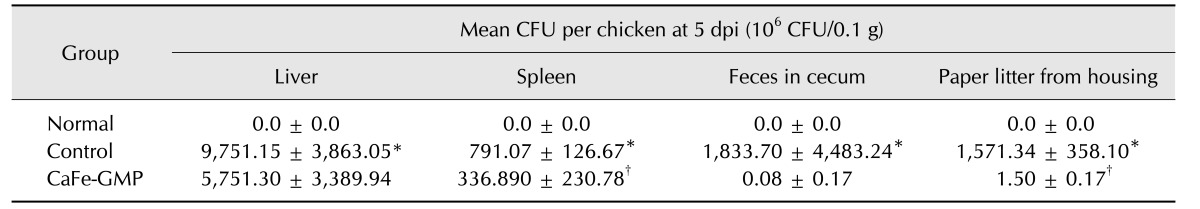
Surviving chickens from each group were sacrificed at 5 dpi. The liver, spleen, and feces from the cecum were aseptically collected and tested to determine whether Salmonella Gallinarum used for the challenge could be re-isolated from organs of the experimental chickens. The liver, spleen, and feces samples were negative for Salmonella Gallinarum in the normal group. In the control group, bacteria were re-isolated at 5 dpi from the liver, spleen, and cecum, indicating a systemic infection had occurred. As shown in Table 5, the numbers of Salmonella Gallinarum colony forming units in the CaFe-GMP additive group were considerably lower at 5 dpi in the liver (45.7%, p < 0.06), spleen (57.4%, p < 0.006), and feces from the cecum (96.6%, p < 0.057) than those in the control group. In particular, Salmonella Gallinarum detection was significantly cleared in excreted feces on sterilized paper litter from the housing units of the CaFe-GMP additive group (99.9%, p < 0.001%) at 5 dpi when compared with that of the control group.
Avian systemic salmonellosis is primarily caused by the Salmonella Gallinarum strain and results in the development of fowl typhoid disease [7]. Fowl typhoid can be acute or chronic and usually results in a high mortality rate [2]. Symptoms include diarrhea, intestinal and womb bleeding, and enlargement of the liver and spleen.
Antibiotics may be used as feed supplements to improve physical performance by suppressing subclinical disease challenge in industrial animals [8]. However, there is worldwide concern regarding the overuse of antibiotics because of the development of multi-antibiotic resistant bacteria and resistance genes and their spread from animals to humans through consumption of food products that contain antibiotic residues [16]. Hence, there is an urgent need to identify natural-origin feed supplements that can reduce antibiotic use. Several studies have demonstrated that prevention of Salmonella colonization in chickens can be achieved by feeding with prebiotics, such as saccharides, organic acid, and inorganic minerals [142425283738].
Mineral additives in poultry feed are needed for bird survival and for efficient poultry production. The mineral requirements of laying hens are well reported [22], but information on trace mineral requirements and their related effects on various aspects such as immune function, resistance to disease, growth, and carcass composition is limited. In this study, we evaluated the effect of a CaFe-GMP feed supplement on the health and egg quality in layers experimentally infected with Salmonella Gallinarum.
Our results demonstrated that administering 0.16% CaFe-GMP as a feed additive had a beneficial effect on body weight in layers after Salmonella Gallinarum infection. It has been reported that mice on an iron-deficient diet gained less weight and ate less [32], suggesting that chickens fed a diet with an iron additive may exhibit less weight loss during intestinal inflammation by Salmonella Gallinarum infection.
Salmonella Gallinarum causes a high mortality rate in fowl [9]. However, in the present study, mortality did not occur in the Salmonella Gallinarum-infected CaFe-GMP additive group during the experimental period. Moreover, clinical signs of an infection were delayed, and the severity of related symptoms was lower in the CaFe-GMP additive group than in the control group. In the control group, there was severe, creamy whitish diarrhea observed from 4 dpi onwards, whereas in the CaFe-GMP additive group there were few typical clinical symptoms of Salmonella Gallinarum infection observed. In addition, the CaFe-GMP additive group had no fatalities. These results are similar to previous results showing that parenteral administration of complexed iron can enhance survival in chicks infected with Salmonella Gallinarum endotoxin (LPS) [30].
With regard to feed intake, the amount of feed consumed in the control group abruptly decreased, supporting results reported by Kim et al. [17]. In the Salmonella Gallinarum-infected CaFe-GMP additive group, there were no significant changes in feed consumption during the experimental period. Moreover, our results showed that the CaFe-GMP additive group displayed no significant change in growth performance.
Infection of fowl by Salmonella Gallinarum results in a decline in egg production rate and broken eggs [1131]. Interestingly, the infected CaFe-GMP additive group retained a normal egg production rate and broken eggs were not observed during the experimental period. Similarly, others have shown that dietary supplementation with a Fe-soy proteinate effectively improved egg quality [28], and dietary supplementation of an organic mineral mixture increased the bioavailability of minerals compared to that from inorganic sources [37]. CaFe-GMP supplementation may protect against the penetration of Salmonella spp. into the oviduct of layers and, therefore, could increase economic profits by maintaining egg production and hatchability in Salmonella-infected layers.
Salmonella infection in chickens occurs at all ages and is characterized by severe hepatomegaly and splenomegaly accompanied by anemia, septicemia, and a liver with a bronzing aspect [29]. Gross changes were observed in the organs of the control group, mainly in the liver, which was green-yellowish in color, larger, and more friable than that in normal layers. There was also spleen congestion and splenomegaly observed in the control group. In the CaFe-GMP additive group, all live chickens exhibited normal organ appearance.
We also examined whether Salmonella Gallinarum could be re-isolated, post-challenge, from organs of the experimental chickens. In the control group, bacteria were re-isolated at 5 dpi from the liver, spleen, and feces from the cecum, which indicated the occurrence of an infection. The numbers of Salmonella Gallinarum in all tested tissues of the CaFe-GMP additive group were significantly lower than those in the control group. Moreover, mortality in the CaFe-GMP additive group was delayed compared to that in the control group. Several reports have demonstrated that dietary iron intake lessens intestinal inflammatory responses and the pathology of enteric infection by foodborne bacterial pathogens, such as the Salmonella enterica serovar Typhimurium in mice [1834]. In addition, iron deficiency has been associated with an impaired immune response, but it can also substantially increase resistance against intracellular pathogens, probably due to an increase in nutritional immunity [35]. Because dietary iron status can affect the immune response, it is likely that the array of antimicrobial defenses secreted from the intestinal mucosa is also affected. Additionally, in the present study, necropsy revealed that the CaFe-GMP additive group had milder and less severe abnormal gross lesions and pathological changes than those observed in the control group, indicating that the CaFe-GMP feed supplement produced bacterial clearance as well as resistance against Salmonella Gallinarum.
Our findings suggest that adding 0.16% CaFe-GMP to the diet of layers can improve clinical symptoms of Salmonella Gallinarum, reduce diarrhea, and result in mild and muted abnormal gross pathological changes in liver and spleen. In addition, CaFe-GMP feed supplementation enabled bacterial clearance from internal organs and maintained egg laying production in layers experimentally infected with Salmonella Gallinarum. Consequently, a CaFe-GMP feed supplement may be useful in preventing salmonellosis in the poultry industry.
Acknowledgments
This research was supported by the High Value-added Food Technology Development Program (113024-3), Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
References
1. Barrow PA. Salmonella infections: immune and non-immune protection with vaccines. Avian Pathol. 2007; 36:1–13. PMID: 17364505.

2. Barrow PA, Freitas Neto OC. Pullorum disease and fowl typhoid—new thoughts on old diseases: a review. Avian Pathol. 2011; 40:1–13. PMID: 21331943.

3. Basnet HB, Kwon HJ, Cho SH, Kim SJ, Yoo HS, Park YH, Yoon SI, Shin NS, Youn HJ. Reproduction of fowl typhoid by respiratory challenge with Salmonella Gallinarum. Avian Dis. 2008; 52:156–159. PMID: 18459315.
4. Boruta A, Swierczewska E, Glebocka K, Nollet L. Trace organic minerals as a replacement of inorganic sources for layers: effects on productivity and mineral excretion. In : World Poultry Science Association, Proceedings of the 16th European Symposium on Poultry Nutrition; 26-30 August 2007; Strasbourg, France.
5. Bovee-Oudenhoven IM, Lettink-Wissink MLG, Van Doesburg W, Witteman BJM, Van Der Meer R. Diarrhea caused by enterotoxigenic Escherichia coli infection of humans is inhibited by dietary calcium. Gastroenterology. 2003; 125:469–476. PMID: 12891550.
6. Bovee-Oudenhoven IM, Termont DS, Weerkamp AH, Faassen-Peters MA, Van der Meer R. Dietary calcium inhibits the intestinal colonization and translocation of Salmonella in rats. Gastroenterology. 1997; 113:550–557. PMID: 9247475.

7. Chappell L, Kaiser P, Barrow P, Jones MA, Johnston C, Wigley P. The immunobiology of avian systemic salmonellosis. Vet Immunol Immunopathol. 2009; 128:53–59. PMID: 19070366.

8. Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002; 13:7–27. PMID: 12212945.

9. de Oliveira GH, Berchieri A Jr, Fernandes AC. Experimental infection of laying hens with Salmonella enterica serovar Gallinarum. Braz J Microbiol. 2005; 36:51–56.
10. de Paiva JB, Penha Filho RAC, Argüello YMS, da Silva MD, Gardin Y, Resende F, Berchieri A Jr, Sesti L. Efficacy of several Salmonella vaccination programs against experimental challenge with Salmonella Gallinarum in commercial brown layer and broiler breeder hens. Rev Bras Cienc Avic. 2009; 11:65–72.
11. Faitarone ABG, Pavan AC, Mori C, Batista LS, Oliveira RP, Garcia EA, Pizzolante CC, Mendes AA, Sherer MR. Economic traits and performance of Italian quails reared at different cage stocking densities. Rev Bras Cienc Avic. 2005; 7:19–22.

12. Gyles CL. Antimicrobial resistance in selected bacteria from poultry. Anim Health Res Rev. 2008; 9:149–158. PMID: 19102788.

13. Hong SS, Jeong J, Lee J, Kim S, Min W, Myung H. Therapeutic effects of bacteriophages against Salmonella Gallinarum infection in chickens. J Microbiol Biotechnol. 2013; 23:1478–1483. PMID: 23801253.
14. Janssens GPJ, Millet S, Van Immerseel F, De Buck J, Hesta M. The impact of prebiotics and salmonellosis on apparent nutrient digestibility and Salmonella Typhimurium var. Copenhagen excretion in adult pigeons (Columba livia domestica). Poult Sci. 2004; 83:1884–1890. PMID: 15554066.
15. Jung BG, Ko JH, Lee BJ. Dietary supplementation with a probiotic fermented four-herb combination enhances immune activity in broiler chicks and increases survivability against Salmonella Gallinarum in experimentally infected broiler chicks. J Vet Med Sci. 2010; 72:1565–1573. PMID: 20675965.
16. Kamphues J. [Antibiotic growth promoters for the view of animal nutrition]. Berl Munch Tierarztl Wochenschr. 1999; 112:370–379. German. PMID: 10598354.
17. Kim MS, Yoon YS, Seo JG, Lee HG, Chung MJ, Yum DY. A study on the prevention of salmonella infection by using the aggregation characteristics of lactic Acid bacteria. Toxicol Res. 2013; 29:129–135. PMID: 24278639.

18. Kortman GA, Mulder ML, Richters TJ, Shanmugam NK, Trebicka E, Boekhorst J, Timmerman HM, Roelofs R, Wiegerinck ET, Laarakkers CM, Swinkels DW, Bolhuis A, Cherayil BJ, Tjalsma H. Low dietary iron intake restrains the intestinal inflammatory response and pathology of enteric infection by food-borne bacterial pathogens. Eur J Immunol. 2015; 45:2553–2567. PMID: 26046550.

19. Maciel MP, Saraiva EP, de Fátima Aguiar É, Ribeiro PAP, Passos DP, Silva JB. Effect of using organic microminerals on performance and external quality of eggs of commercial laying hens at the end of laying. Rev Bras Zootec. 2010; 39:344–348.

20. Mastroeni P, Chabalgoity JA, Dunstan SJ, Maskell DJ, Dougan G. Salmonella: immune responses and vaccines. Vet J. 2001; 161:132–164. PMID: 11243685.

21. Murray MJ, Murray AB, Murray MB, Murray CJ. The adverse effect of iron repletion on the course of certain infections. Br Med J. 1978; 2:1113–1115. PMID: 361162.

22. National Research Council. Nutrient Requirements of Poultry: Ninth Revised Edition, 1994. Washington, DC: National Academies Press;1994. p. 3–18.
23. Nyakeriga AM, Troye-Blomberg M, Dorfman JR, Alexander ND, Bäck R, Kortok M, Chemtai AK, Marsh K, Williams TN. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004; 190:439–447. PMID: 15243915.

24. Patterson JA, Burkholder KM. Application of prebiotics and probiotics in poultry production. Poult Sci. 2003; 82:627–631. PMID: 12710484.

25. Rasschaert G, Michiels J, Tagliabue M, Missotten J, De Smet S, Heyndrickx M. Effect of organic acids on Salmonella shedding and colonization in pigs on a farm with high Salmonella prevalence. J Food Prot. 2016; 79:51–58. PMID: 26735029.
26. Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006; 367:133–143. PMID: 16413877.

27. Schaible UE, Kaufmann SHE. Iron and microbial infection. Nat Rev Microbiol. 2004; 2:946–953. PMID: 15550940.

28. Seo YM, Shin KS, Rhee AR, Chi YS, Han J, Paik IK. Effects of dietary Fe-soy proteinate and MgO on egg production and quality of eggshell in laying hens. Asian-Australas J Anim Sci. 2010; 23:1043–1048.

29. Shivaprasad HL. Fowl typhoid and pullorum disease. Rev Sci Tech. 2000; 19:405–424. PMID: 10935271.

30. Smith IM, Licence ST, Hill R. Haematological, serological and pathological effects in chicks of one or more intravenous injections of Salmonella Gallinarum endotoxin. Res Vet Sci. 1978; 24:154–160. PMID: 653115.
31. St Louis ME, Morse DL, Potter ME, DeMelfi TM, Guzewich JJ, Tauxe RV, Blake PA. Salmonella enteritidis Working Group. The emergence of grade A eggs as a major source of Salmonella enteritidis infections. New implications for the control of salmonellosis. JAMA. 1988; 259:2103–2107. PMID: 3279240.
32. Tompkins GR, O’Dell NL, Bryson IT, Pennington CB. The effects of dietary ferric iron and iron deprivation on the bacterial composition of the mouse intestine. Curr Microbiol. 2001; 43:38–42. PMID: 11375662.

34. Wang L, Johnson EE, Shi HN, Walker WA, Wessling-Resnick M, Cherayil BJ. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J Immunol. 2008; 181:2723–2731. PMID: 18684963.

35. Weiss G. Modification of iron regulation by the inflammatory response. Best Pract Res Clin Haematol. 2005; 18:183–201. PMID: 15737884.

36. Wigley P, Hulme S, Powers C, Beal R, Smith A, Barrow P. Oral infection with the Salmonella enterica serovar Gallinarum 9R attenuated live vaccine as a model to characterise immunity to fowl typhoid in the chicken. BMC Vet Res. 2005; 1:2. PMID: 16221297.

37. Yenice E, Mizrak C, Gültekin M, Atik Z, Tunca M. Effects of organic and inorganic forms of manganese, zinc, copper, and chromium on bioavailability of these minerals and calcium in late-phase laying hens. Biol Trace Elem Res. 2015; 167:300–307. PMID: 25800653.

38. Ziprin RL, Elissalde MH, Hinton A Jr, Beier RC, Spates GE, Corrier DE, Benoit TG, DeLoach JR. Colonization control of lactose-fermenting Salmonella Typhimurium in young broiler chickens by use of dietary lactose. Am J Vet Res. 1991; 52:833–837. PMID: 1883086.
Fig. 1
Effect of dietary supplementation of organic minerals on the macroscopic change in liver in layer groups post-challenge. Representative images from the (A) normal group, (B) control group and (C) CaFe-GMP group. Scale bars = 5 cm.
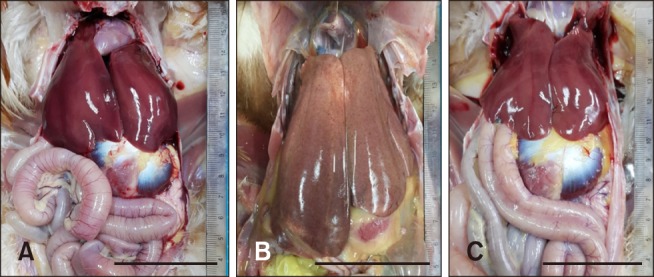
Table 1
Composition of the basal diet
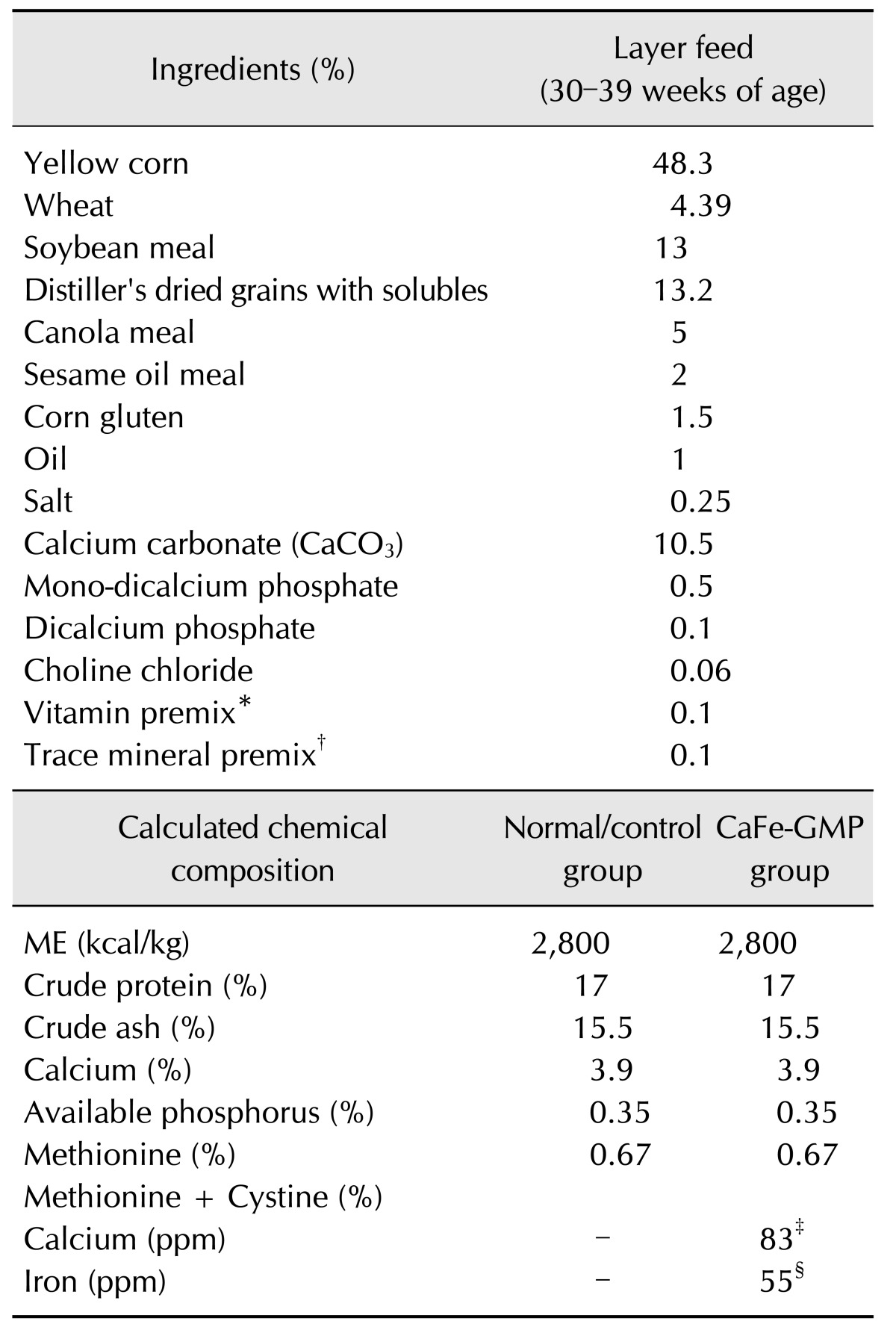
*Supplied per kg of diet: vitamin A 13,000,000 IU, vitamin D3 4,000,000 IU, vitamin B1 3,000 mg, vitamin B2 7,000 mg, vitamin B6 5,000 mg, vitamin B12 20 mg, lysin 3,000 mg, methionine 1,000 mg, tryptophane 700 mg, pantothenic acid 12,000 mg, niacin 50,000 mg, biotin 150 mg, and folic acid 1,500 mg. †Supplied per kg of diet: Iron 46 mg, zinc 78 mg, copper 7 mg, Selenium 0.1 mg, Manganese 87 mg, iodin 1.3 mg, and chromium 0.12 mg. ‡Guanosine 5′-monophosphate (GMP)-chelated calcium (organic mineral form). §Guanosine 5′-monophosphate (GMP)-chelated iron (organic mineral form). ME, metabolizable energy.
Table 2
Effect of dietary supplementation of organic minerals on the body weight in layer groups, post-challenge
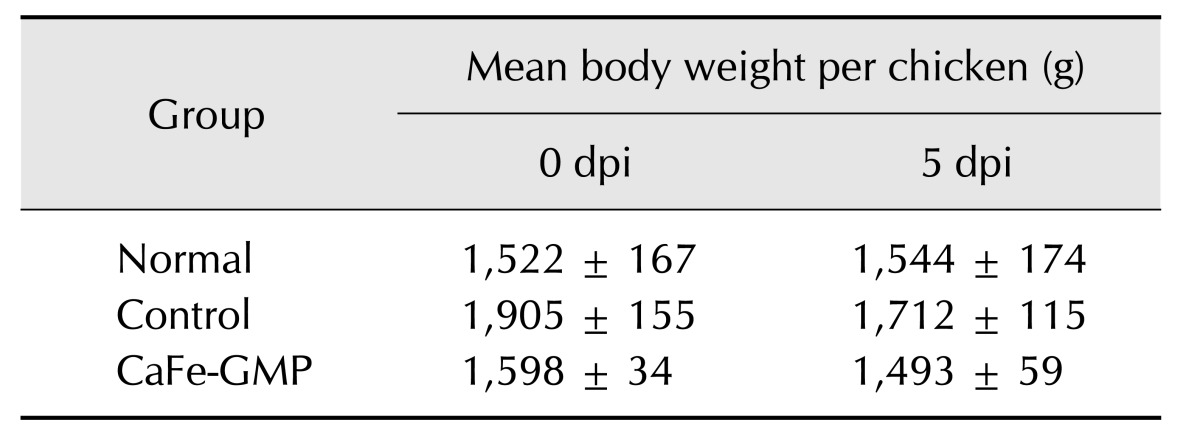
Table 3
Effect of dietary supplementation of organic minerals on performance parameters and egg production characteristics in layer groups, post-challenge




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download