Abstract
The incidence of lung cancer has rapidly increased and cancer patients at a later cancer stage frequently suffer from unbearable cancer-associated pain. However, the pathophysiology of lung cancer pain has not been fully described due to a lack of appropriate animal models. This study was designed to determine the effect of Lewis lung carcinoma (LLC) cell inoculation on formalin-induced pain behavior and spinal Fos expression in C57BL/6 mice. LLC cells (1.5 × 105, 2.5 × 105, 3.0 × 105 or 5.0 × 105) were inoculated into back or peri-sciatic nerve areas. Back area inoculation was adopted to determine the effect of cancer cell circulating factors and the peri-sciatic nerve area was used to evaluate the possible effects of cancer cell contacting and circulating factors on formalin-induced pain. At postinoculation day 7, LLC cell (5.0 × 105) inoculations in both back and peri-sciatic nerve area significantly increased formalin-induced paw-licking time and spinal Fos expression over those in cell-media-inoculated (control) mice. Enhanced pain behavior and spinal Fos expression were significantly suppressed by ibuprofen pretreatment (250 mg/kg). The results of this study suggest that LLC cell circulating factors and inflammatory responses may be critical in enhancing pain sensation in the early stage of lung cancer cell inoculation.
Cancer is a critical life-threatening disease and the human population with cancer has been rapidly increasing. As a tumor develops and/or spreads to other body areas, a patient frequently suffers from severe cancer-related pain. Since the precise mechanism of cancer-associated pain is poorly understood, effective, mechanism-based treatments are difficult to implement. In addition, the lack of a proper animal model for cancer pain research has limited the pharmaceutical development of new analgesics.
The formalin test has been commonly used to examine the efficacy of analgesic drugs in various animal species [14]. Peripheral injection of formalin produces typical biphasic pain behavior and elevates spinal Fos expression [612]. Pain behavior in the first phase occurs as a consequence of direct chemical noxious stimulation while that of the second phase is mediated by the formalin-induced central sensitization and/or peripheral inflammation [2].
This study was designed to investigate the effect of Lewis lung carcinoma (LLC) cell inoculation on formalin-induced pain behavior and spinal Fos expression. For the determination of the effect of circulating factors associated with inoculated cancer cells, LLC cells were subcutaneously administrated into the back area, whereas they were inoculated into the peri-sciatic nerve area in separate groups of mice to determine the roles of circulating and nerve-contacting factors. In addition, we treated with ibuprofen to determine the possible role of inflammatory responses in LLC-enhanced and formalin-induced pain behaviors and in spinal Fos expression.
Male C57BL/6 mice weighing 28 to 32 g were purchased from Samtaco Bio Korea (Korea) and used for this study. All animal experimentation adhered to the policy of the Chungnam National University regarding the use and care of animals. Animals were housed in a standard environment with a 12 h light/dark cycle, a stable room temperature (maintained between 20 and 25℃), and 40% to 60% humidity. Food and water were given freely throughout the investigation.
The LLC cells were purchased from the American Type Culture Collection (USA). This tumor originated spontaneously as a carcinoma of the lung of C57BL/6 mice. The LLC cells were cultured in Dulbecco's modified Eagle medium (DMEM, Sigma-Aldrich, USA) with 10% fetal bovine serum (Gibco, USA) and 5% antibiotic-antimycotic (Gibco) at 37℃ in an atmosphere of 5% CO2 and 95% air and passaged weekly. For LLC administration, cells were centrifuged for 3 min at 200 × g; subsequently, the sediment was suspended in media and used for the inoculations.
Mice were anesthetized by an intraperitoneal injection of Zoletil 50 (5 mg/kg, tiletamine HCl + zolazepam HCl; Virbac Laboratories, France) and Rompun (0.75 mg/kg, xylazine HCl; Bayer Korea, Korea). Inoculation of various concentrations of LLC (1.5 × 105, 2.5 × 105, 3.0 × 105 or 5.0 × 105 cells in a volume of 100 µL) was performed into the back area (subcutaneous; lumbar 4–6) or the right peri-sciatic nerve area (intramuscular) in each mouse. Control group mice were inoculated (intramuscular) with cell media solution in the peri-sciatic nerve area.
The formalin test was carried out as described in our previous report [7]. At 7 days after cell media or LLC inoculation, formalin (1%, 20 µL) was subcutaneously injected into the plantar surface of the hind paw. Following intraplantar injection of formalin, the animals were immediately placed in an acrylic observation chamber (40 cm high, 20 cm diameter), and their behaviors recorded for 30 min by using a video camera. Following the videotaping, paw-licking time (in seconds per each 5 min increment) was calculated by two experienced investigators, blinded to the experimental conditions. For analysis, formalin-induced total paw-licking time was divided into two phases: first (0–10 min) and second (10–40 min) phases. To examine the possible effect of the inflammatory response on LLC-enhanced and formalin-induced pain, ibuprofen (250 mg/kg; Sigma-Aldrich) was intraperitoneally injected 30 min before formalin injection. Vehicle for ibuprofen was prepared with 10% (v/v) DMSO in physiological saline solution. The dose of ibuprofen was selected as previously described [16].
Immunohistochemistry and image analysis of Fos expression was performed by applying our previously described method [8]. Two hours after intraplantar formalin injection, the mice were deeply anesthetized and transcardially perfused with cold saline followed by a fixative (4% paraformaldehyde in PBS). The lumbar spinal cord was removed immediately after the perfusion and post-fixed in a same fixative for 12 h. Samples of the spinal cord were cryoprotected by submersion in 30% sucrose in PBS (pH 7.4) for 12 h. Frozen serial sections (35 µm thickness) were cut through the lumbar 4-6 spinal cord by using a cryotome (Leica, Germany). After the elimination of endogenous peroxidase activity with 0.3% hydrogen peroxide in PBS, sections were pre-blocked with 1% normal goat serum and 0.1% Triton X-100 in PBS. Free-floating sections were incubated in rabbit anti-Fos antiserum (1:10,000, Calbiochem; MilliporeSigma) at 4℃ overnight. Next, the sections were incubated in horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1,000; Abcam, USA) for 90 min at room temperature. Finally, visualization was performed using 3,3′-diaminobenzidine (Sigma-Aldrich) and sections were mounted on silane-coated slides. The slides were then dried, dehydrated in ethanol (70%–100% gradually), cleared in xylene, and cover-slipped.
Determination of each spinal segment was performed by applying a method described previously [1]. Individual sections were examined by microscopy (Axio Scope A1; Zeiss, Germany) and images captured by using a digital camera (Axi℃am MRm; Zeiss). Image analysis software (Image J; National Institutes of Health, USA) was used to examine the number of Fos-like immunoreactive (FLI) neurons. In order to maintain a constant threshold for each image and to compensate for subtle variability in the immunostaining, we only counted neurons that were at least 50% darker than the average gray level of each image. After adjusting the threshold image, individual neurons were considered to be specifically Fos labeled when a total pixel area of between 10 and 30 pixels was detected. Quantitation procedure was performed blindly with regard to the experimental condition of each animal. Cased on cytoarchitectonic criteria, the following gray matter regions were selected for analysis: (1) superficial dorsal horn (SDH; laminae I–II); (2) nucleus proprius (NP; laminae III–IV); and (3) neck of dorsal horn (NECK; laminae V–VI).
Data are expressed as mean ± SEM values. The statistical significance of differences between control and treated groups was determined by applying two-tailed Dunnett's test following one-way analysis of variance (ANOVA). A p value less than 0.05 was considered to be statistically significant.
As shown in Fig. 1, LLC cell media treatments did not significantly change formalin-induced pain levels from that of formalin-treated control mice. In the second paw-licking phase (10–40 min), only the highest LLC cell number (5.0 × 105) inoculated into back or peri-sciatic nerve areas significantly increased formalin-induced paw-licking time (panel D in Fig. 1). Formalin-induced pain in the second phase was not affected by back or peri-sciatic nerve area inoculations of lower LLC cell numbers (1.5 × 105, 2.5 × 105, or 3.0 × 105).
The enhanced formalin-induced paw-licking time during the second phase after the 5 × 105 LLC inoculation was markedly attenuated by pretreatment with ibuprofen (250 mg/kg, i.p.) as shown in Fig. 2. Ibuprofen and media treatment did not affect formalin-induced pain. In addition, a lower ibuprofen dose (50 mg/kg) did not affect the LLC-enhanced formalin pain (data not shown).
Photographs of Fos expression in each group are presented in Fig. 3 and the immunochemical analysis results are shown in Fig. 4. The LLC cell inoculations into the back and peri-sciatic nerve areas markedly increased the formalin-induced Fos expression in the SDH (panels B and C in Fig. 3). Pretreatment with ibuprofen (250 mg/kg) significantly suppressed the LLC-enhanced and formalin-induced spinal Fos expressions in the back and peri-sciatic nerve groups as shown panels E and F in Fig. 3 and Fig. 4. Ibuprofen and media treatment did not affect formalin-enhanced spinal Fos expression (panel D in Fig. 3 and Fig. 4).
Cancer patients in the final cancer stage frequently suffer from unbearable pain. Cancer-related pain can be caused by one or several factors including inflammatory responses, ischemia due to insufficient blood supply, tumor growth-induced nerve compression, and/or the toxic effect of chemotherapeutic agents [3]. However, mechanism-based treatments for this type of pain have been difficult to develop because the pathophysiology of cancer-associated pain has not been fully described. Lung cancer is a leading cause of cancer-associated death and one of the most commonly occurring types of cancer worldwide [5]. Similar to other types of cancer, unbearable pain is a major factor decreasing the quality of life in lung cancer patients [41015]. Although previous studies have focused on the development of an animal model mimicking the neuropathic pain of cancer patients, only a limited number of cancer animal models, such as those for fibro- and osteosarcoma, have been reported [11]. To date, no animal model for the study of lung cancer pain has been reported, and the lack of such animal models limits the pharmaceutical development of new analgesics for the treatment of lung cancer pain. In this regard, this study was undertaken to develop a new animal model for the study of lung cancer pain.
In this study, we adopted a formalin-induced pain mouse model to determine the possible effect of lung cancer cells on inflammatory pain by inoculating LLC cells into C57BL/6 mice. To determine the roles of circulating or contacting factors, we treated LLC cells into mouse back (circulating factors) or peri-sciatic nerve (both contacting and circulating factors) areas. As shown in Fig. 1, the highest LLC treatment level (5.0 × 105 cells) in both back and peri-sciatic nerve area significantly increased formalin-induced pain behavior in the second paw-licking phase, and there was no statistical difference in LLC pain enhancement between the back and peri-sciatic nerve groups. This result suggests that circulating factors from inoculated LLC may be critical for pain enhancement and supports the results in a previous report that showed that lung cancer patients suffer from unbearable pain in multiple sites [15]. In a further experiment in this study, LLC-enhanced formalin-induced pain behavior was significantly reduced by pretreatment with ibuprofen (250 mg/kg). In addition, pretreatment with ibuprofen significantly decreased the LLC- and formalin-enhanced Fos expression at the spinal lumbar level. A previous report suggested that the formalin-induced pain behavior in the second phase may be produced by a different mechanism related to the concentration of formalin [16]. Only 5% formalin, not 1%, significantly produced plasma extravasation in the injected paw and anti-inflammatory agents such as dexamethasone and ibuprofen elicited both anti-inflammatory and antinociceptive effects on the higher formalin-induced responses. Thus, it can be suggested that the antinociceptive effect of ibuprofen, as used in this study, produced its effect via the suppression of LLC-induced inflammation rather than via formalin, and the inflammatory response in the early phase following cancer cell inoculation may be critical factor for its pronociceptive effect [13]. In another animal model for cancer pain, gabapentin showed sufficient pain relief on melanoma-induced allodynia and hyperalgesia, but an aspirin-like anti-inflammatory drug, diclofenac, did not produce analgesia in the same animal model [9]. In that report, gabapentin and diclofenac were administered between 14 and 20 days after cancer cell inoculation; in contrast, we treated ibuprofen on day 7 after LLC cell treatment. Such differences may indicate that cancer cell-induced pain mechanisms (i.e., inflammatory or neuropathic) may differ depending on the time of cancer cell inoculation.
In conclusion, we designed this study to develop a new animal model for assessing lung cancer pain by inoculating LLC cells into C57BL/6 mice. The injected LLC, at a cell number of 5.0 × 105, enhanced formalin-induced pain behavior and spinal Fos expression. These enhanced pain responses were significantly inhibited by pretreatment with the anti-inflammatory drug, ibuprofen (250 mg/kg). The animal model develop in this study may be useful in the development of new pharmaceutical analgesic drugs to reduce or remove pain associated with lung cancer.
Figures and Tables
Fig. 1
Effect of Lewis lung carcinoma (LLC) cell inoculation on formalin-induced pain behavior. LLC cells were administered into mouse back (Back) or peri-sciatic nerve (Sciatic) areas at four different inoculated cell numbers: 1.5 × 105 (A), 2.5 × 105 (B), 3.0 × 105 (C), and 5.0 × 105 (D). At 7 days postinoculation, formalin-induced first phase (0–10 min) pain was not affected in all groups of mice. Only the highest number of injected LLC cells (5.0 × 105) significantly increased formalin-induced late phase (10–40 min) pain in Back and Sciatic areas (D). Data are presented as mean ± SEM values. *p < 0.05 and **p < 0.001 in comparison to media + formalin group.

Fig. 2
Effect of pretreatment with ibuprofen (Ibu, 250 mg/kg, intraperitoneally) on Lewis lung carcinoma (LLC; 5.0 × 105) cell-enhanced formalin pain behavior. Formalin test was performed 7 days after the inoculation of LLC into back (Back) or peri-sciatic nerve (Sciatic) areas. Ibu treatment 30 min prior to the formalin injection significantly decreased late phase (10–40 min) LLC-enhanced formalin pain behavior during the second phase (10–40 min after formalin injection). Data are presented as mean ± SEM values. *p < 0.05 in comparison to vehicle (V)-treated control group.

Fig. 3
Representative photomicrographs illustrating Lewis lung carcinoma (LLC) cell-enhanced and formalin-induced Fos expressions in the ipsilateral spinal cord dorsal horn of lumbar 4–6 spinal segments. (A) formalin-injected control; (B) LLC cells (5.0 × 105) injected into back area + formalin injection; (C) LLC cells (5.0 × 105) injected into peri-sciatic nerve area + formalin injection; (D) media only injected into peri-sciatic nerve area + formalin injection + ibuprofen pretreatment (250 mg/kg, intraperitoneally); (E) LLC cells injected into back + formalin injection + ibuprofen pretreatment; (F) LLC cells injected into peri-sciatic nerve area + formalin injection + ibuprofen pretreatment. SDH, superficial dorsal horn (laminae I–II); NP, nucleus of proprius (laminae III–IV); NECK, dorsal horn neck (laminae V–VI). Scale bar = 200 µm.
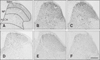
Fig. 4
Effect of pretreatment with ibuprofen (Ibu, 250 mg/kg, intraperitoneally) on Lewis lung carcinoma (LLC) cell-enhanced and formalin-induced expression of Fos-like immunoreactive (FLI) neurons in the spinal dorsal horn. LLC inoculation into back (Back) or peri-sciatic nerve (Sciatic) areas significantly increased the number of FLI neurons in each segment of the spinal dorsal horn; that is, superficial dorsal horn (SDH; laminae I–II), nucleus of proprius (NP; laminae III–IV) and dorsal horn neck (NECK; laminae V–VI). Ibu treatment occurred 30 min prior to formalin injection and suppressed the LLC- and formalin-induced increase in the number of FLI neurons. ***p < 0.001.

Acknowledgments
This research was supported by Chungnam National University and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (grant No. HI15C0007) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2011-0014381).
References
1. Abbadie C, Besson JM. Chronic treatments with aspirin or acetaminophen reduce both the development of polyarthritis and Fos-like immunoreactivity in rat lumbar spinal cord. Pain. 1994; 57:45–54.

2. Abbott FV, Franklin KB, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995; 60:91–102.

3. Bennett GJ. Pathophysiology and animal models of cancer-related painful peripheral neuropathy. Oncologist. 2010; 15(Suppl 2):9–12.

4. Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain. 2012; 153:359–365.

5. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917.

6. Hwang HJ, Kim P, Kim CJ, Lee HJ, Shim I, Yin CS, Yang Y, Hahm DH. Antinociceptive effect of amygdalin isolated from Prunus armeniaca on formalin-induced pain in rats. Biol Pharm Bull. 2008; 31:1559–1564.

7. Kim HW, Kwon YB, Roh DH, Yoon SY, Han HJ, Kim KW, Beitz AJ, Lee JH. Intrathecal treatment with σ1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br J Pharmacol. 2006; 148:490–498.

8. Kim KW, Kim HW, Li J, Kwon YB. Effect of bee venom acupuncture on methamphetamine-induced hyperactivity, hyperthermia and Fos expression in mice. Brain Res Bull. 2011; 84:61–68.

9. Kuraishi Y, Iida Y, Zhang HW, Uehara S, Nojima H, Murata J, Saiki I, Takahata H, Ouchi H. Suppression by gabapentin of pain-related mechano-responses in mice given orthotopic tumor inoculation. Biol Pharm Bull. 2003; 26:550–552.

10. Mystakidou K, Parpa E, Tsilika E, Pathiaki M, Galanos A, Vlahos L. Comparison of pain quality descriptors in cancer patients with nociceptive and neuropathic pain. In Vivo. 2007; 21:93–97.
11. Pacharinsak C, Beitz A. Animal models of cancer pain. Comp Med. 2008; 58:220–233.
12. Roh DH, Yoon SY. Sigma-1 receptor antagonist, BD1047 reduces nociceptive responses and phosphorylation of p38 MAPK in mice orofacial formalin model. Biol Pharm Bull. 2014; 37:145–151.

13. Sasamura T, Nakamura S, Iida Y, Fujii H, Murata J, Saiki I, Nojima H, Kuraishi Y. Morphine analgesia suppresses tumor growth and metastasis in a mouse model of cancer pain produced by orthotopic tumor inoculation. Eur J Pharmacol. 2002; 441:185–191.

14. Sufka KJ, Watson GS, Nothdurft RE, Mogil JS. Scoring the mouse formalin test: validation study. Eur J Pain. 1998; 2:351–358.

15. Wilkie DJ, Huang HY, Reilly N, Cain KC. Nociceptive and neuropathic pain in patients with lung cancer: a comparison of pain quality descriptors. J Pain Symptom Manage. 2001; 22:899–910.




 Citation
Citation Print
Print


 XML Download
XML Download