Abstract
This is the first case report to describe the tumor regressive effect of systemic human neural stem cell (NSC)/5-fluorocytosine (5-FC) therapy on canine metastatic lung tumor. The therapeutic effects appeared approximately two weeks after 5-FC administration. Thoracic radiographs revealed a reduced number of lung nodules and decreased nodule size. However, there were no significant antitumor effects on primary lesions in abdominal organs. In conclusion, human NSC/5-FC prodrug therapy can secure patient quality of life with the same or more therapeutic effects and fewer side effects than other recommended chemotherapies.
Hemangiosarcoma (HSA) is a malignant form of neoplasm of vascular endothelium commonly observed in dogs. There are two hypotheses regarding the origin of HSA, mutated vascular endothelial lining cells or multipotential stem cells prior to hemangioblast [7]. It has been reported in human studies that exposure to thorium dioxide, arsenicals, vinyl chloride, and androgen increases the potential for HSA progression [4]. Dogs beyond middle-age are most commonly affected, and HSA occurs more often in breeds such as Golden Retrievers, Labrador Retrievers, and German Shepherds [10]. The spleen is the most frequent primary site, while other sites include the right atrium, skin, subcutis, and liver [2]. Hematogenous metastasis is frequent and transabdominal implantation by tumor rupture can occur. The liver, omentum, mesentery, and lungs are the most common metastatic sites [2].
Splenectomy is the primary treatment choice for canine HSA and should be aggressively performed to remove locally invaded tissues. Owing to the high metastatic rate of canine HSA, and the poor outcome associated with surgery alone, adjuvant chemotherapy or other novel therapies are suggested in most cases. The recommended chemotherapy protocol is a combination of vincristine, doxorubicin, and cyclophosphamide [2].
Many other therapeutic modalities can be used to treat HSA in dogs. Introducing genes into a patient's cells is an emerging method of treatment for HSA. Additionally, destroying cancer cells by providing suicidal genes with tumor-tropic neural stem cells (NSCs) and prodrugs was suggested in a previous study in mice [5]. Human stem cells can be transplanted into dogs without inducing rejection or tumors [89]. The discovery of inherent tumor-tropic properties of NSCs provides a new approach to overcome difficulties in delivering the therapeutic agent to the neoplasm [61215]. Cancer gene therapy is based on providing a gene that encodes an enzyme, which, in turn, transforms an inert prodrug into a toxic product. It has been revealed that cytosine deaminase (CD), which can convert 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU)-expressing human NSCs, was useful in examining the tumor reducing effect on mice model [114]. Cytosine deaminase enzyme can convert the prodrug 5-FC into the activated form 5-FU and 5-FU kill adjacent unmodified cancer cells [14].
This is the first case report that describes the tumor regressive effect of a combination therapy involving human NSCs (hNSCs) engineered to express the CD gene and the prodrug 5-FC on metastatic lung tumor in a dog with HSA.
An 8-year-old, male, Maltese dog weighing 3.4 kg was referred to the university teaching hospital. Before being referred, the patient was diagnosed with hypoglycemia, hypothermia, anemia, and neutrophilia at a veterinary clinic. The patient's mucous membrane was observed to be notably pale, the urine dark yellow, and the patient had shown anorexia and depression for about 10 days.
The complete blood count (CBC) and serum chemistry results were hematocrit 21.5%, white blood cell 24,344/µL, platelet 94.8 × 103/µL, glucose 142 mg/dL, albumin 2.3 g/dL, blood urea nitrogen 62.7 mg/dL and alkaline phosphatase 206 IU/L. Abdominal ultrasonographic examination demonstrated free fluid in the abdominal cavity, a round-shaped splenic mass, and a hypoechoic lesion of the liver. The splenic mass size was 3.5 cm × 2.8 cm. Abdominocentesis was performed and cytology of the abdominal fluid revealed an increased neutrophil count and epithelial cells with neoplastic changes such as anisocytosis, anisokaryosis, and macronucleoli. To confirm the number and region of masses in the abdominal cavity and other abnormalities, a computed tomography scan was performed. A multifocal liver mass was located in the right medial, left lateral, and caudate lobes and a splenic mass was present with severe peritoneal fluid. There was no evidence of pulmonary metastasis.
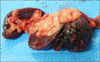
Before surgery, the patient was stabilized with blood transfusion, fluid therapy, and antibiotics. The patient was premedicated for general anesthesia with atropine (Atropine sulfate, 0.02 mg/kg, subcutaneous; Jeil Pharmaceutical, Korea), cefotaxime (Cefotaxime sodium, 30 mg/kg, intravenous [IV]; Wooridul Pharmaceutical, Korea) and tramadol (Maritrol, 3 mg/kg, IV; Jeil Pharmaceutical). Anesthesia was induced with propofol (Provive 1%, 5 mg/kg, IV; Myungmoon Pharm, Korea) and maintained with isoflurane. Throughout surgery, the patient was transfused and received intravenous 2.5% dextrose with 0.45% NaCl solution and dopamine solution (Dopamine HCl, 5 µg/kg/min, CRI; Huons, Korea). After abdominal wall incision, about 200 mL of bloody free fluid in the peritoneal cavity were removed. The mass located in the splenic tail had a round shape and was 3.5 cm in diameter. The splenic artery and vein were ligated and the spleen was removed (Fig. 1). Partial hepatic lobectomy of the left medial hepatic, caudate, and right lateral hepatic lobes that included the multifocal mass was performed. The maximum dimension of the hepatic mass was approximately 4 cm. The resected spleen and liver masses were submitted for histopathological examination; the diagnosis was HSA.
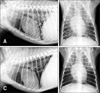
Following surgery, the patient was medicated with 0.5 mg/kg prednisolone (Solondo; Yuhan, Korea) and 10 mg/kg cimetidine (Cimetidine Tab; Taiguk Pharm, Korea) administered in pill form. The patient's vitality and appetite were favorable for one month. However, 30 days after surgery the patient had a cough and a routine radiograph revealed lung nodules of 3 to 10 mm, which were presumed to reflect metastasis of HSA (panels A and B in Fig. 2). Additional ultrasonography showed a right medial hepatic lobe mass and a mesenteric mass.
It was decided to treat the patient with combined hNSCs/5-FC therapy. The hNSCs (1.0 × 107) were diluted with 0.9% N/S 1.5 mL and injected intravenously through a cephalic vein. The hNSCs were engineered to express the CD gene in the laboratory at the Division of Neurology, University of British Columbia, Canada [5]. Starting one week after hNSCs injection, 5-FC was administered weekly for 4 weeks. After the second 5-FC injection, thoracic radiography, abdominal ultrasonography, CBC, and serum chemistry analyses were conducted to monitor the patient's conditions every week for up to 5 weeks.
Therapeutic effects of hNSCs/5-FC therapy appeared approximately two weeks after prodrug (5-FC) administration. Thoracic radiographs revealed a reduced number and size of lung masses (panels C and D in Fig. 2). Tumor regressive effects were remarkable in the pulmonary metastatic region, though there were no significant anticancer effects on the abdominal mass. Almost no side effects related to traditional chemotherapy using doxorubicin and cyclophosphamide such as myelosuppression, gastrointestinal irritation, and depression were observed during hNSCs/5-FC treatment. Patient's vitality and appetite were relatively good until postoperative day 105, when the patient died.
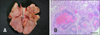
After death, major organs in the abdomen, thorax, and calvarium were evaluated for metastasis through necropsy. The cause of death was tumor rupture and bleeding in the liver. The liver, kidney, adrenal gland, mesenteric fat, lung, right atrium, and brain were affected by HSA metastasis. Grossly visible dark-red metastatic lesions with a size of 1 to 3 mm were detected in the lung at necropsy (panel A in Fig. 3). Microscopically, lung nodules with central hemorrhage and necrosis was well circumscribed by connective tissues (panel B in Fig. 3).
In the present case, canine splenic and hepatic HSA was treated with splenectomy and partial hepatic lobectomy. Suicide gene therapy using hNSCs transduced with E. coli expressing CD was performed as a postoperative adjuvant therapy. As NSCs can trace disseminated tumor cells, they were used to deliver a therapeutic agent by acting as vehicles to the tumor [5]. 5-FC and 5-FU are small, highly water soluble molecules that penetrate well into most body sites, including peritoneal fluid. This combination treatment strategy led to significant tumor regression of the pulmonary metastasis, but not of the abdominal tumor. Metastatic lung nodules would have increased substantially in size and number if the dog had not been treated with hNSCs/5-FC therapy; actually, the nodules at autopsy were smaller than those observed upon radiography. Injected hNSCs migrate to the neoplastic region though blood flow; thus, cells injected through the cephalic vein reached the lungs. The majority of intravenously injected mesenchymal stem cells (MSCs) are trapped in the lungs upon first passage, and the MSCs are relocated, particularly to liver and spleen, after 24 hours [3]. These findings suggest that most of the hNSCs injected were concentrated in the pulmonary metastatic region; therefore, the antitumor effects of the hNSCs/5-FC approach were not observed in the intraabdominal and intracranial tumors.
Survival time following surgery alone to treat HSA ranges from 19 to 86 days, and less than 10% of the patients survive for 12 months [1113]. Surgery and adjuvant chemotherapy with vincristine, methotrexate, and cyclophosphamide results in a mean survival time of 117 days. However, these chemotherapeutic agents have different degrees of side effects such as neutropenia, lethargy, anorexia, vomiting, diarrhea, and fever. In this patient, instead of traditional chemotherapy, hNSCs/5-FC therapy was applied one month after surgery. In a previous study of hNSCs and 5-FC prodrug therapy, MDA-MB-435 (a human breast cancer cell line) tumor-bearing mice received hNSCs and 5-FC. In that study, the tumor sizes were reduced by up to 60% and hNSCs were shown to have the capacity to migrate selectively to brain metastasis, where they significantly reduced the tumor burden after administration of the 5-FC [5].
It should be noted that this study had several limitations. First, HSA metastasis to other tissues such as the brain, kidney, or heart was not monitored during treatment; therefore, the treatment's therapeutic efficacy to reduce the size and number of metastasis is unknown in these organs. Second, implanted hNSCs could not be tracked in this case; therefore, we do not know how many cells migrated and were active in reducing tumor size in lungs. Finally, a major limitation is that only one dog was studied.
In conclusion, it can be suggested that hNSCs/5-FC therapy can secure the quality of patient's life with the same or a greater level of therapeutic effects and much lower side effects than those from traditional chemotherapy. Additional clinical studies are needed to elucidate the effects of hNSCs/5-FC on canine metastatic lung tumor.
Figures and Tables
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2015R1A6A1A04020885).
References
1. Aboody KS, Najbauer J, Schmidt NO, Yang W, Wu JK, Zhuge Y, Przylecki W, Carroll R, Black PM, Perides G. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006; 8:119–126.

2. Clifford CA, Mackin AJ, Henry CJ. Treatment of canine hemangiosarcoma: 2000 and beyond. J Vet Intern Med. 2000; 14:479–485.

3. Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014; 5:148.

4. Falk H, Herbert J, Crowley S, Ishak KG, Thomas LB, Popper H, Caldwell GG. Epidemiology of hepatic angiosarcoma in the United States: 1964-1974. Environ Health Perspect. 1981; 41:107–113.

5. Joo KM, Park IH, Shin JY, Jin J, Kang BG, Kim MH, Lee SJ, Jo M, Kim SU, Nam DH. Human neural stem cells can target and deliver therapeutic genes to breast cancer brain metastases. Mol Ther. 2009; 17:570–575.

6. Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, Cho BK, Kim M, Menon LG, Black PM, Carroll RS. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006; 12:5550–5556.

7. Lamerato-Kozicki AR, Helm KM, Jubala CM, Cutter GC, Modiano JF. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol. 2006; 34:870–878.

8. Lee SH, Chung YN, Kim YH, Kim YJ, Park JP, Kwon DK, Kwon OS, Heo JH, Kim YH, Ryu S, Kang HJ, Paek SH, Wang KC, Kim SU, Yoon BW. Effects of human neural stem cell transplantation in canine spinal cord hemisection. Neurol Res. 2009; 31:996–1002.

9. McMahill BG, Borjesson DL, Sieber-Blum M, Nolta JA, Sturges BK. Stem cells in canine spinal cord injury – promise for regenerative therapy in a large animal model of human disease. Stem Cell Rev. 2015; 11:180–193.

10. Moe L, Gamlem H, Dahl K, Glattre E. Canine neoplasia – population-based incidence of vascular tumours. APMIS Suppl. 2008; (125):63–68.

11. Prymak C, McKee LJ, Goldschmidt MH, Glickman LT. Epidemiologic, clinical, pathologic, and prognostic characteristics of splenic hemangiosarcoma and splenic hematoma in dogs: 217 cases (1985). J Am Vet Med Assoc. 1988; 193:706–712.
12. Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, Black PM, Aboody KS, Carroll RS. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005; 7:623–629.

13. Wood CA, Moore AS, Gliatto JM, Ablin LA, Berg RJ, Rand WM. Prognosis for dogs with stage I or II splenic hemangiosarcoma treated by splenectomy alone: 32 cases (1991-1993). J Am Anim Hosp Assoc. 1998; 34:417–421.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download