Abstract
The efficacies of six commercial disinfectants were evaluated by using Salmonella enterica serovar Typhimurium under simulated natural conditions such as sub-zero temperature, short disinfecting time, and surface type (uneven or smooth). We used a suspensionmodel test to determine the disinfecting efficacy under varying contact times (1, 5, 10, and 30 min) and temperatures (25℃, 4℃, 0℃, and −10℃). The bactericidal effect according to surface structure was measured by using a carriermodel test at 25℃ and −10℃. The effective concentrations of each disinfectant were fixed to give a disinfecting effect within a short time (< 1 min) at 25℃ and −10℃. The suspension model results revealed that bactericidal efficacy significantly dropped at low temperature for most of the disinfectants used; a sodium dichloroisocyanurate product showed the strongest efficacy. In the carrier test, bacterial load on a wooden surface was more difficult to remove than that on a stainless-steel surface. The results show that commercial disinfectant products vary in their disinfecting efficacy, which is affected by several field factors including temperature, contact time, and carrier material. Environmental conditions and surface type for disinfection should be considered prior to selecting an optimal disinfectant in the field.
Salmonellosis is an important public health issue. Salmonella (S.) enterica serovar Typhimurium and S. enterica serovar Enteritidis are responsible for most cases of human salmonellosis with over 80% of these infections being caused by poultry products, such as eggs and poultry meat [1924].
Once chickens are exposed to Salmonella, the poultry flock is colonized quickly. The infected chickens shed Salmonella through feces, which leads to meat contamination during slaughtering. Adult laying hens can be asymptomatic carriers that continually produce contaminated eggs [19]. Therefore, a farm-level Salmonella eradication program is needed to eliminate Salmonella infection. Disinfection is a very important component of the biosecurity program designed by the poultry industry [20].
Adequate and correct disinfection procedures can reduce the incidence of diseases and their transmission. The kinetics of disinfection are altered by several environmental factors, including organic load, temperature, and contact time [16]. Hence, when a disinfection program is adopted in a farm or industrial setting, many factors must be considered for successful decontamination [4].
In this study, changes in the efficacy, induced by different environmental factors such as temperature, contact time, and surface type, of disinfection of six disinfectants used in Korean farms were evaluated. Four contact times (1, 5, 10, and 30 min) and four temperature conditions (25℃, 4℃, 0℃, and −10℃) were investigated by using suspension tests. Bactericidal efficacy by surface type (wood and stainless steel) was evaluated by using carrier tests. Moreover, the concentration of each disinfectant was set to produce a disinfectant effect within a short time (< 1 min) at 25℃ and −10℃ to assess disinfectants in warm and cold seasons, respectively.
S. Typhimurium strain ATCC 13311, used as the test organism, was incubated in nutrient broth (Oxoid, USA) overnight at 37℃. The inoculum turbidity was determined by an absorbance of 0.005 at 600 nm by using a micro-ELISA plate reader (SoftMax Pro, USA).
Six disinfectants commonly used in farms were assessed. Each disinfectant was diluted in hard water, by dissolving 0.139 g magnesium chloride hexahydrate (Junsei Chemical, Japan) and 0.305 g calcium chloride (Sigma-Aldrich, USA) in de-ionized water, diluting to 1,000 mL and autoclaving at 121℃ for sterilization. The hard water was stored in the refrigerator for no longer than 1 month. The working concentrations of each disinfectant were prepared based on the manufacturers' recommendations, which were also approved by the Animal and Plant Quarantine Agency (QIA), Korea, and are detailed in Table 1.
Two carriers with different surface structure types were used, birch wood piece (1.5 cm × 1.5 cm, square) and stainless steel (2 cm diameter, AISI 304; Posco, Korea). Before use, carriers were washed twice in deionized water and autoclaved at 121℃ for 15 min.
Four different reaction temperatures (25℃, 4℃, 0℃, and −10℃) and contact times (1, 5, 10, and 30 min) were investigated. For low-temperature application, diluted disinfectants were kept on ice for 30 min at 0℃ and then maintained in a refrigerator set at −10℃ for 20 min.
Suspension (4 mL) containing the test organism (> 107 CFU/mL, absorbance with ELISA reader = 0.005) was added to 96 mL of 5% yeast extract (Oxoid, USA) solution, and 2.5 mL of this mixture was then inoculated with 2.5 mL of each disinfectant. After the appropriate contact time, 1 mL of the solution was neutralized with 9 mL of Dey-Engley neutralizing solution (Difco, USA) for 5 min. The number of residual viable organisms was determined by determining plate counts on 3M Petrifilm Aerobic Count Plates (3M, USA). The concentration establishment test was performed in the same way as the suspension test, except that the contact times used were 30 sec, 1 min, and 5 min.
Equivalent volumes of 3% bovine serum albumin (Sigma, USA) solution and test inoculum suspensions were mixed, and 100 µL of this mixture were inoculated onto each carrier. Each carrier in 6-well plates (Nunc, Denmark) was placed on a clean bench with airflow on and light off for 60 to 80 min. In the 25℃ group, carriers and disinfectants were maintained at room temperature. In the −10℃ group, dried carriers were placed in a refrigerator at −10℃ for 10 min. Disinfectants were kept on ice before use. Each carrier was inoculated with 200 µL of each disinfectant, and after 1 and 5 min, the carriers were transferred to 50-mL tubes containing 10 mL of Dey-Engley neutralizing broth (Difco). The bacterial cells from the carriers were detached by full-speed vortexing for 3 min. The residual surviving bacteria were determined by plate counts on 3M Petrifilm aerobic count plates (3M).
We considered a 5 log10 reduction (99.999%) compared to the control which was not treated by disinfectants to quantify the recovered Salmonella by using the plate count method. At 25℃, 2.5% citric acid (CA) was effective (> 5 log10 reduction) after a 10 min contact; however, it did not inactivate S. Typhimurium at low temperatures (4℃, 0℃, or −10℃) despite a 30 min contact time. At a reaction time of 30 min, 2.4, 2.24, and 1.7 reductions were recorded at 4℃, 0℃, and −10℃, respectively (panel A in Fig. 1). Although disinfection was slightly more rapid than that of CA at 25℃ and 4℃, a similar pattern was observed in 0.03% CA + 0.02% quaternary ammonium compounds (QACs) at 0℃ and −10℃. At 4℃, the CA + QAC combination showed 3.31 and 3.76 reductions in the 5 and 10 min reactions; however, 7.8 log10 reduction (complete bactericidal activity) occurred in the 30 min reaction (panel B in Fig. 1). In 0.5% potassium peroxymonosulfate (MPS), 7.7, 5.1, 4.87, and 4.45 reductions were observed at 25℃, 4℃, 0℃, and −10℃, respectively, at the 10min contact time (panel C in Fig. 1). At the 30 min contact time, 7.8 log10 was observed in all temperature conditions, while 1% MPS + 0.1% sodium dichloroisocyanurate (NaDCC) showed faster efficacy (over 5 log10 reduction attained at 5 min) than MPS only (panel D in Fig. 1). In suspension tests, 0.3% NaDCC was the most effective and rapid-acting agent, with 100% bactericidal activity at all temperature conditions and within a short time (1 min) (Fig. 1). In contrast, 0.1% glutaraldehyde (GA) was the most temperature-sensitive agent. With the exception of 25℃ at10 and 30 min, bacteria were hardly inactivated (panel F in Fig. 1). The levels of reduction by 0.1% GA were 1.47, 1.12, and 1.18 at 4℃, 0℃, and −10℃, respectively, after 30 min.
Most disinfectants were more effective on the stainless-steel surface than on the wooden surface (p < 0.05). Complete reduction of bacteria was defined as 5.1 log10 reduction on stainless steel and 5.09 log10 reduction on wood, while a 4 log10 reduction was defined as effective. For adequate disinfection of stainless steel at 25℃, a 5 min contact time was needed; whereas a > 5 min contact time was required for wood disinfection at the same temperature. At −10℃, the stainless-steel carrier test revealed that most disinfectants were able to completely decontaminate. S. Typhimurium; the exception being 0.1% GA with a 5 min contact time. However, not all disinfectants were effective against dried S. Typhimurium on wood at −10℃. Similar to the suspension test results, 0.1% GA showed 2.84 and 0.2564 log10 reductions on stainless steel and wood, respectively, at −10℃. The oxidizing agents, namely, MPS, MPS + NaDCC, and NaDCC, were generally more effective than acid agents (Fig. 2).
We evaluated the efficacy of using 2× and 4× the manufacturers' recommended concentrations of each disinfectant at 25℃ and −10℃ for 0 sec, 30 sec, 1 min, and 5 min. A complete bactericidal effect was observed after 30 sec of 10% CA (4×) treatment at 25℃, whereas 4× CA required 1 to 5 min for adequate efficacy (> 5 log10 reduction) at −10℃ (panel A in Fig. 3). The 2× and 4× concentrations of CA + QACs showed complete bactericidal effect at the time of treatment at 25℃, whereas only the 4× concentration of CA + QACs (0.8% CA + 0.4% QACs) reduced S. Typhimurium completely at the time of treatment at −10℃. Adequate bactericidal efficacy from 2× CA + QACs (0.4% CA + 0.2% QACs) required > 30 sec (panel B in Fig. 3). S. Typhimurium was adequately inactivated by 1% MPS administered for 0.5 to 1 min at 25℃. At −10℃, 1% MPS showed sufficient bactericidal effect after 1 to 5 min (panel C in Fig. 3). However, 2× MPS + NaDCC (2% MPS + 0.2% NaDCC) failed to provide > 5 log10 reduction within 1 min at −10℃ (panel D in Fig. 3). S. Typhimurium was inactivated completely and immediately by 1% NaDCC (3×) at −10℃ (panel E in Fig. 3). Although it was ineffective at −10℃, even after 5 min at 0.1% and 0.2% glutaraldehyde (2×), 0.4% glutaraldehyde (4×) showed > 5 log10 reductions at 1 min at 25℃ and at 5min at −10℃ (panel F in Fig. 3).
Disinfectants are used for disease prevention and control in a variety of fields [13]. Many livestock and poultry farms have introduced farm hazard analysis and critical control point (HACCP) programs that deem it mandatory to install foot dips in front of premises and disinfection facilities (including wheel dips) at farm entrances [20].
The efficacy of disinfectants is affected by disinfectant type, mode of application, exposure time, natural microbial population, surfaces, and temperature. Thus, we evaluated changes in the efficacy of six disinfectants under different conditions, including four contact times (1, 5, 10, and 30 min), four temperatures (25℃, 4℃, 0℃, and −10℃), and two surface types to reflect field environments.
Although foodborne salmonellosis outbreaks mainly occur in summer (> 20℃), Salmonella are able to survive > 550 days at −20℃ and 4℃ with no significant reduction [6]. Further, dried Salmonella cells on paper discs can survive for 2 years at 4℃ [22]. The efficacy of most chemical disinfectants is negatively affected at low temperatures [4]. Due to the lack of scientific literature on the efficacy of disinfectants at extreme cold temperature, we conducted suspension tests under various temperatures, including below zero conditions, with S. Typhimurium, a representative test microorganism for disinfectant efficacy standards in QIA [1]. The disinfectants used in this study completely inactivated S. Typhimurium in 30 min at 25℃. However, modes of application, such as dipping, spraying, misting, or fumigation are not generally performed in the field for 30 min. because of evaporation and inactivation of the disinfectants by organic matter and soil [15]. Hence, an ideal disinfectant should be effective within a very short time, especially at low environmental temperatures. This study found that most disinfectants were unable to inactivate S. Typhimurium within 5 min at 25℃; hence, when disinfectants are applied at 25℃, the disinfection process should last at least 5 to 10 min and include soaking the surface being disinfected with the disinfectant solution. The 2.5% CA and 0.03% CA + 0.02% QACs showed inadequate efficacies at 4℃, 0℃, and −10℃, even with a prolonged contact time of 10 min. These two chemicals are commonly used in farms and food processing industries because of their safety levels compared to those of other chemicals [2]. The environmental temperature in milk or meat processing factories are maintained under 15℃ in the work areas and under 4℃ in the storage areas [8]. Moreover, the human infectious dose of S. Typhimurium is around 2 log10 [7]. Therefore, it is necessary to determine the optimum concentration and contact time of disinfectants for the actual ambient temperature in the work area in order to achieve complete decontamination of S. Typhimurium. The activity of CA is enhanced by the presence of anionic detergents like QACs. Therefore, combination agents can be applicable in different fields, including processing industries, after establishing the appropriate concentrations at low temperatures or by expanding the exposure times [12].
At 0℃ and −10℃, 0.5% MPS and 1% MPS + 0.1% NaDCC were effective against S. Typhimurium at a > 10 min contact time, while 0.3% NaDCC showed complete S. Typhimurium inactivation, regardless of contact time or temperature. Although the liquid disinfectant is effective after 10 min at low temperatures, the temperature of the liquid is rapidly lowered in the field immediately after spraying during the cold season. Therefore, the disinfectant exposure time ends up being significantly shorter [15]. Thus, we conducted additional experiments to determine the effective concentrations for immediate disinfection at below zero temperatures.
A 4× of the manufacturer's recommended concentration of CA (10%) there was incomplete reduction of bacteria at1 min at −10℃, whereas 4× concentrations of CA + QACs (0.8% CA + 0.4% QACs) showed immediate, complete reduction (i.e., 7.8 log10 reduction) at −10℃. S. Typhimurium was also inactivated by treatment with 2× concentrations of CA + QACs (0.4% CA + 0.2% QACs) for > 30 sec at −10℃. With the exception of 0.1% GA, the MPS-based disinfectants were able to show immediate, adequate bactericidal efficacy at 4× concentrations, which was the recommended concentration for use at −10℃. Among the six disinfectants, GA was unable to sufficiently inactivate S. Typhimurium at −10℃, and S. Typhimurium was only effectively reduced by 4× GA (0.4%) at −10℃ after 5 min. Although most disinfectants were able to quickly (1 min) inactivate S. Typhimurium when their concentrations were increased, high concentrations of chemicals increase the risk of environmental pollution and risks to the person applying the disinfectant [12]. Aldehydes (formaldehyde and glutaraldehyde) are generally considered effective disinfectants at the farm level [51011]. However, glutaraldehyde fumes are notably irritating and toxic to the mucous membranes, particularly those of the respiratory tract. Respiratory tract irritation is observed at 0.3 ppm concentrations [2]. In addition, a cross-sectional study of Dutch pig farmers showed that the use of disinfectants, including aldehydes, was an important etiological factor in chronic respiratory health effects [17]. In addition, high concentrations of or long exposure times to oxidizing agents and acids can be corrosive to metal surfaces [2]. Therefore, temperature range and exposure time should be considered when choosing an appropriate disinfectant.
Suspension tests are easy to perform; however, they have certain limitations. Pathogens adhere to equipment surfaces through organic or cellular debris [1418]. Carrier tests are more relevant for predicting the activity of chemical disinfectants under field conditions [9]. Two kinds of carriers with different surface types were selected, namely, stainless steel (2 cm diameter, AISI 304B; Posco) and wood (1.5 cm × 1.5 cm square), to represent farm environments. The results for the stainless-steel carrier at 25℃ showed that disinfectant efficacy depended on the disinfectant's property, such as whether it was a fast-acting agent or not. Although most disinfectants did not inactivate to a > 4 log10 reduction within 1 min, oxidizing agents such as MPS, MPS + NaDCC, and NaDCC can act quickly. At both 25℃ and −10℃, stainless steel showed a > 4 log10 reduction at 5 min contact time with most disinfectants except glutaraldehyde. With a 5 min contact time on wood at 25℃, most disinfectants, except MPS + NaDCC, showed a < 4 log10 reduction. The difference in disinfectant efficacy between stainless steel and wood was greatest at −10℃. On wood, all six disinfectants were ineffective against dried S. Typhimurium after 5 min. A study by Yilmaz et al. [25] revealed that disinfection of porous surfaces is more difficult than that of non-porous surfaces, as porous surfaces impede the removal of the particles through cleaning or resuspension in the disinfectant solution. In this study, we found similar patterns of disinfection differences between dried bacteria on wood and the less porous stainless steel. Thus, cleaning should be performed prior to disinfection to overcome the efficacy difference of surface materials. Cleaning is regarded as a crucial procedure to eliminate infectious pathogens. After a single wipe with water and liquid soap, influenza virus has shown an approximate 4 log10 reduction [21]. However, successful elimination of Salmonella from poultry by cleaning and disinfection is labor intensive and costly, as well, it requires attention with respect to selection and application of the disinfectant [23]. A field trial showed that cleaning and disinfection programs have failed in 60% of 60 commercial laying houses due to cleaning difficulty associated with structures intrinsic to laying houses [3]. Therefore, appropriate disinfectants that will penetrate into organic and porous material in farms should be chosen.
This study aimed to evaluate efficacy changes among six disinfectants under various conditions including temperature, contact time, and surface type. The efficacy of most disinfectants was decreased by low temperatures, short contact times, and porous surfaces. Thus, environmental temperature, disinfection duration, and target surface should be considered for successful disinfection infield situations. Disinfectants that are intended for veterinary applications are assessed for efficacy by standard testing methods supported by national bodies, such as the Animal and Plant Quarantine Agency in Korea and our study used standard testing methods. As stated above, the practice of effective disinfection varies considerably according to the method of application, such as spray, mist, or fumigation; thus, various test protocols are required to reflect field conditions. Further studies are required to determine the ideal length of disinfectant contact time at low temperatures.
Figures and Tables
Fig. 1
Evaluation of Salmonella Typhimurium bactericidal activity of six disinfectants by suspension testing at four different contact times and temperatures. Complete reduction of bacteria was defined as ≥ 7.8 log10 reduction, while a > 5 log10 reduction was considered effective. (A) 2.5% citric acid. (B) 0.03% CA + 0.02% QACs. (C) 0.5% MPS. (D) 1% MPS + 0.1% NaDCC. (E) 0.3% NaDCC. (F) 0.1% GA.
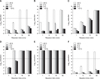
Fig. 2
The effect of each disinfectant against dried Salmonella Typhimurium on wood and stainless steel at 25℃ or −10℃ for 1 min or 5 min. Complete reduction of bacteria was defined as 5.1 log10 reduction on stainless steel and 5.09 log10 reduction on wood, while a 4 log10 reduction was defined as effective. Error bars indicate standard deviations of the means. (A) 25℃ for 1 min. (B) 25℃ for 5 min. (C) −10℃ for 1 min. (D) −10℃ for 5 min. **Significant differences between the results from stainless steel and wood (p < 0.01).
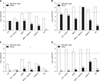
Fig. 3
Evaluation of bactericidal activity for short contact times by different concentrations of each disinfectant at 25℃ and −10℃. Complete reduction of bacteria was defined as ≥ 7.8 log10 reduction and > 5 log10 reduction was considered effective reduction. (A) Citric acid. (B) Citric acid + QACs. (C) MPS. (D) MPS + NaDCC. (E) NaDCC. (F) Glutaraldehyde.

Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bio-industry Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant No. 20110211).
References
1. Animal and Plant Quarantine Agency (KR). Biocides Efficacy Test against Bacteria. Notice 2013-34 (Mar. 23 2013).
2. Carling PC, Wormser GP. Antisepsis, disinfection and sterilization: types, action and resistance by Gerald E. McDonnell Wahington, DC: American Society for Microbiology Press, 2007. 378 pp., illustrated. Clin Infect Dis. 2007; 45:1251–1252.

3. Carrique-Mas JJ, Marin C, Breslin M, McLaren I, Davies R. A comparison of the efficacy of cleaning and disinfection methods in eliminating Salmonella spp. from commercial egg laying houses. Avian Pathol. 2009; 38:419–424.

4. De Benedictis P, Beato MS, Capua I. Inactivation of avian influenza viruses by chemical agents and physical conditions: a review. Zoonoses Public Health. 2007; 54:51–68.

5. Gradel KO, Sayers AR, Davies RH. Surface disinfection tests with Salmonella and a putative indicator bacterium, mimicking worst-case scenarios in poultry houses. Poult Sci. 2004; 83:1636–1643.

6. Hiramatsu R, Matsumoto M, Sakae K, Miyazaki Y. Ability of Shiga toxin-producing Escherichia coli and Salmonella spp. to survive in a desiccation model system and in dry foods. Appl Environ Microbiol. 2005; 71:6657–6663.

7. Kang S, Jang A, Lee SO, Min JS, Kim IS, Lee M. Effect of organic acids on microbial populations and Salmonella typhimurium in pork loins. Asian-Australas J Anim Sci. 2003; 16:96–99.

8. Koleci X, Quinn PJ, Çela M, Malaj Z. The place of disinfection in the control of infectious diseases. Albanian J Nat Tech Sci. 2007; 12:139–156.
9. Kuda T, Iwase T, Yuphakhun C, Takahashi H, Koyanagi T, Kimura B. Surfactant-disinfectant resistance of Salmonella and Staphylococcus adhered and dried on surfaces with egg compounds. Food Microbiol. 2011; 28:920–925.

10. Marin C, Hernandiz A, Lainez M. Biofilm development capacity of Salmonella strains isolated in poultry risk factors and their resistance against disinfectants. Poult Sci. 2009; 88:424–431.

11. McLaren I, Wales A, Breslin M, Davies R. Evaluation of commonly-used farm disinfectants in wet and dry models of Salmonella farm contamination. Avian Pathol. 2011; 40:33–42.

12. Møretrø T, Heir E, Nesse LL, Vestby LK, Langsrud S. Control of Salmonella in food related environments by chemical disinfection. Food Res Int. 2012; 45:532–544.

13. Møretrø T, Midtgaard ES, Nesse LL, Langsrud S. Susceptibility of Salmonella isolated from fish feed factories to disinfectants and air-drying at surfaces. Vet Microbiol. 2003; 94:207–217.

14. Møretrø T, Vestby LK, Nesse LL, Storheim SE, Kotlarz K, Langsrud S. Evaluation of efficacy of disinfectants against Salmonella from the feed industry. J Appl Microbiol. 2009; 106:1005–1012.

15. Payne JB, Kroger EC, Watkins SE. Evaluation of disinfectant efficacy when applied to the floor of poultry grow-out facilities. J Appl Poult Res. 2005; 14:322–329.

16. Pinto F, Maillard JY, Denyer SP. Effect of surfactants, temperature, and sonication on the virucidal activity of polyhexamethylene biguanide against the bacteriophage MS2. Am J Infect Control. 2010; 38:393–398.

17. Preller L, Heederik D, Boleij JSM, Vogelzang PFJ, Tielen MJM. Lung function and chronic respiratory symptoms of pig farmers: focus on exposure to endotoxins and ammonia and use of disinfectants. Occup Environ Med. 1995; 52:654–660.

18. Sattar SA, Springthorpe VS, Adegbunrin O, Abu Zafer A, Busa M. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J Virol Methods. 2003; 112:3–12.

19. Shah DH, Zhou XH, Addwebi T, Davis MA, Orfe L, Call DR, Guard J, Besser TE. Cell invasion of poultry-associated Salmonella enterica serovar Enteritidis isolates is associated with pathogenicity, motility and proteins secreted by the type III secretion system. Microbiology. 2011; 157:1428–1445.

20. Stringfellow K, Anderson P, Caldwell D, Lee J, Byrd J, McReynolds J, Carey J, Nisbet D, Farnell M. Evaluation of disinfectants commonly used by the commercial poultry industry under simulated field conditions. Poult Sci. 2009; 88:1151–1155.

21. Tuladhar E, Hazeleger WC, Koopmans M, Zwietering MH, Beumer RR, Duizer E. Residual viral and bacterial contamination of surfaces after cleaning and disinfection. Appl Environ Microbiol. 2012; 78:7769–7775.

22. Uesugi AR, Danyluk MD, Harris LJ. Survival of Salmonella enteritidis phage type 30 on inoculated almonds stored at –20, 4, 23, and 30℃. J Food Prot. 2006; 69:1851–1857.

23. Wales A, Breslin M, Davies R. Assessment of cleaning and disinfection in Salmonella-contaminated poultry layer houses using qualitative and semi-quantitative culture techniques. Vet Microbiol. 2006; 116:283–293.

24. Wong HS, Townsend KM, Fenwick SG, Maker G, Trengove RD, O'Handley RM. Comparative susceptibility of Salmonella Typhimurium biofilms of different ages to disinfectants. Biofouling. 2010; 26:859–864.

25. Yilmaz A, Heffels-Redmann U, Redmann T. Evaluation of the virucidal efficacy of two chemical disinfectants against avian influenza virus A at different temperatures. Arch Geflügelk. 2004; 68:50–56.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download