Abstract
The postoperative analgesic effects of firocoxib in ovariohysterectomized cats were observed. Twenty-four cats were divided into 3 groups: control (no medicine), firocoxib-1 (1 mg/kg/day) and firocoxib-3 (3 mg/kg/day). Colorado pain scale scores (CPSS), composite pain scores (CPS), and buccal mucosal bleeding times (BMBT) were recorded in blinded fashion before induction and 2, 5, 8, 24, 30, and 48 h post-operation. The average CPSS (mean ±SEM) over 2 to 48 h post-operation in firocoxib-3 (0.4 ± 0.1) was significantly lower than that of the control (0.7 ± 0.2; p = 0.004), but that of firocoxib-1 (0.5 ± 0.2) was not different from that of the control (p = 0.40). The mean CPS of firocoxib-3 was significantly lower than that of the control at 24 h post-operation (p = 0.04); nonetheless, there was no significant difference in mean CPS between firocoxib-1 and control groups at all intervals. BMBT and body temperature were within normal limits in all groups. However, reversible azotemia was identified in two firocoxib-3 cats at 72 h post-operation. One firocoxib-3 cat vomited once at 48 h post-operation. In conclusion, firocoxib-3 is helpful for postoperative pain control in cats; however, gastrointestinal irritation and renal function side effects may occur.
Analgesic drugs are essential components of postoperative care in veterinary medicine [1]. Opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed for postoperative pain control in small animal patients [1824]. The inhibition of cyclooxygenase (COX) enzymes by NSAIDs prevents transformation of arachidonic acid to prostaglandin or other inflammatory mediators. Thus, NSAIDs are helpful for fever management and inflammation induced by injury [30]. COX-1, found in unremarkable tissues, helps produce prostaglandin, which maintains normal body homeostasis, including gastrointestinal mucosa protection, platelet aggregation, and renal blood flow [1129]. On the other hand, COX-2, induced by injuries, has roles in producing prostaglandins and pain mediators, as well as in amplifying nociceptive pain signaling to the spinal cord and brain [1421]. Side effects of NSAIDs are related to their specificity to inhibit COX enzymes, including COX-1 and COX-2 [2730]. Currently, selective NSAIDs, which inhibit only COX-2, are preferable because they result in fewer side effects on kidneys and platelet aggregation [25] and less gastrointestinal irritation [1527].
Firocoxib, classified as COX-2 selective, has been shown by various studies to have minimal side effects when used for pain control in dogs and horses with osteoarthritis [172328]. Moreover, firocoxib is effective for acute pain control in dogs in the postoperative periods [20]. However, the effects of firocoxib in feline patients are unclear [22]. Cats have lower hepatic glucuronyl transferase activity than that in dogs, which means cats have an inadequate inability to convert drugs into an ineffective form for elimination [1035]. A lower dosage of NSAIDs is generally recommended for use in cats compared to dogs in order to avoid side effects. One study used oral administration of firocoxib for pyrexia prevention in two cats [25]. Pharmacokinetics indicated that the time to maximal plasma concentration was 1 and 4 h, respectively, and eliminated half-life was 8.7 and 12.2 h [25]. The half-maximal inhibitory concentration ratio (the ratio of COX-1 to COX-2 activity) for firocoxib was 58 [25], suggesting that firocoxib is a COX-2 selective NSAID. Although it has been reported that oral firocoxib at a dosage of 0.75 to 3.0 mg/kg in cats helped to reduce fever without side effects [25], the effects of firocoxib when used for pain control in cats remain undescribed.
The purpose of the present study was to investigate the proper dosage of firocoxib for postoperative pain control in cats undergoing ovariohysterectomy. The adverse effects of firocoxib on hemostasis were recorded before each operation by determining buccal mucosal bleeding times. The gastrointestinal and renal toxicities due to the use of firocoxib were assessed over three postoperative days by using clinical signs and blood chemistry.
The study involved female cats undergoing ovariohysterectomy. Pregnant cats and cats with systemic, orthopedic, or neurological problems were excluded from the experiment. Two cats from twenty-six cats that expressed a composite pain score exceeding 8 points at the baseline (38% of full composite pain scores) were considered aggressive and excluded from the experiment. The 24 enrolled cats weighted from 2.5 to 5 kg. (mean ±SEM; 3.4 ± 0.2 kg) and were 6 months to 7 years of age (3.3 ± 0.5 years old). A veterinarian performed physical and blood examinations of the cats before their operation. Each cat in the study was admitted to the hospital for at least 20 h. Food and water were restricted for 8 h before the operation. The investigation conformed to the Guide for the Care and Use of Laboratory Animals of Kasetsart University (approval No. OACKU00458), and informed owner consent was obtained for all cats.
All cats underwent the same anesthetic protocol. Using a catheter placed into the cephalic vein, intravenous fluids (0.9% normal saline solution) were administered to all cats. Anesthesia was induced with propofol, 4 to 6 mg/kg intravenously, without premedication. The anesthesia was maintained with isoflurane and oxygen delivered in a semi-open rebreathing system. Isoflurane, which has hypnosis analgesic effects and is a muscle relaxant to reduce pain during anesthesia was set at 2%, an approximate 1.5 minimal alveolar concentration (MAC) during the operation [1331]. Enrofloxacin (5 mg/kg) was administered as a prophylactic antibiotic by subcutaneous injection. The buccal mucosal bleeding times were recorded before the operation by using a surgical blade to create a 1 cm long 1 mm deep incision in the buccal mucosa. Blood from the incision was blotted using filter paper held near the incision. Ovariohysterectomy was performed via the ventral midline approach using a small incision (approximately 1 cm long) by an experienced surgeon that had been trained for over 5 years in damage control surgery. Average surgical time was 15 ±5 min.
After the operation, the cats were transferred to a recovery room. Each cat was blindly randomized into one of three groups with eight cats per group. Group 1 (control group) received a sugar pill placebo, Group 2 (firocoxib-1 group) received firocoxib at 1 mg/kg [16], and Group 3 (firocoxib-3 group) received firocoxib at 3 mg/kg [25]. Group members were given their respective treatment orally 1 h before anesthesia induction. The treatments were given again at 24 and 48 h post-operation. It should be noted that all treatments were given after recording the pain scores to avoid unintentional effects on pain monitoring.
All cats were observed by the same blinded investigator during the 72 h postoperative period at intervals of 0, 2, 5, 8, 24, 30, 48, and 72 h. Assessment of postoperative pain was performed by using Colorado pain scale scores and composite pain scores. Colorado pain scale scores were determined after reviewing the pain criteria against a 4 point scale in 0.5 increments (Table 1), where 0 is no pain at all and 4 is the worst pain [12]. Composite pain scores [1] ranging from 0 to 21 were assessed using 0 as normal and 21 as the worst pain (Table 2). Composite pain score is the sum of the individual scores from 7 assessments: temperament (0–4); appearance (0–3); body posture (0–2); unprovoked behavior (0–3); interactive behavior (0–4); movement (0–2); and vocalization (0–3).
The threshold for commencing a rescue analgesic procedure (i.e., morphine at 0.3 mg/kg IM) was set at either a Colorado pain scale scores above 2.5 or a composite pain score above 12 at 8 h after surgery or beyond. Cats with pain scores above these limits would immediately receive the rescue analgesic drug and be excluded from the study. The presence of any adverse effects, including vomiting, diarrhea, decreased appetite, lethargy, melena, somnolence, hyperactivity, skin reactions, and constipation were recorded [2632].
Blood was collected before each operation as a baseline sample and at 72 h post-operation to evaluate complete blood count, blood urea nitrogen (BUN), creatinine, alanine aminotransferase (ALT), alkaline phosphatase (ALK), total protein, albumin, and serum globulin.
The average Colorado pain scale scores and composite pain scores in each group were analyzed by using a one-way ANOVA. The difference between two groups was compared by using Dunnett's t-test. The adverse effects in the treatment groups were compared to the control group by using Fisher's exact test. Data are expressed as mean ± SEM values. The level of significance was considered as p < 0.05.
Among the three treatment groups, there were no significant differences by cat age (means: control group, 3.1 ± 0.4 years; firocoxib-1 group, 3.5 ± 0.6 years; firocoxib-3 group, 3.3 ± 0.4 years; p = 0.85) or by weight (means: control group, 3.6 ± 0.3 kg; firocoxib-1 group, 3.3 ± 0.2 kg; firocoxib-3 group, 3.4 ± 0.3 kg; p = 0.58). In addition, average buccal mucosal bleeding times were not different among the three groups (means: control group, 50.6 ± 13.6 sec; firocoxib-1 group, 66.6 ± 13.8 sec; firocoxib-3 group, 46.3 ± 7.4 sec; p = 0.17; Fig. 1). All cats recovered from the anesthetic drug uneventfully.
The average baseline Colorado pain scale scores from the control, firocoxib-1, and firocoxib-3 groups were not significantly different (0.4 ± 0.2, 0.3 ± 0.1, and 0.5 ± 0.2, respectively; p > 0.05; Fig. 2). There were no cats in any group that expressed a Colorado pain scale score exceeding 2.5 points, thus no cat required rescue analgesia during the recovery period. There were no significant differences in mean Colorado pain scale scores (recorded up to 48 h post-operation) among the firocoxib-1, firocoxib-3, and control groups at all intervals (Fig. 2). Interestingly, average Colorado pain scale scores over the 2 to 48 h postoperative period in the firocoxib-3 group (0.4 ± 0.1) was significantly lower than that of the control group (0.7 ± 0.2; p = 0.004; Fig. 3). However, no significant difference was detected between average Colorado pain scale scores over the 2 to 48 h postoperative periods in the firocoxib-1 (0.5 ± 0.2) and control groups (p = 0.40), and no significant difference between average Colorado pain scale scores over the 2 to 48 h postoperative periods in the firocoxib-1 and firocoxib-3 groups (p = 0.38; Fig. 3).
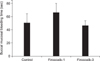
Similar to Colorado pain scale score results, the average baseline composite pain scores from the control, firocoxib-1, and firocoxib-3 groups were not significantly different (3.3 ± 0.7, 3.0 ± 1.1, and 3.4 ± 1.0, respectively; p > 0.05; Fig. 4). Mean composite pain score of the firocoxib-3 group was significantly lower than that of the control group at 24 h post-operation (p = 0.04). The mean composite pain scores of the firocoxib-1 group was lower than that of the control group at 8 h post-operation, although the difference was not significant. While the composite pain score of the firocoxib-1 group was less than that of the control group at 8 h post-operation, the mean composite pain scores of the firocoxib-3 group was less than that of the control group for the entire recovery period. The cats receiving firocoxib-3 and firocoxib-1 returned to normal baseline composite pain scores at 5 h and 30 h post-operation, respectively. Firocoxib-1 tended to be less effective in pain score reduction in the early portion of the recovery period. The average temperature of all three groups at 24 h post-operation was normal with no significant differences among the groups (means: control group, 37.7 ± 0.2℃; firocoxib-1 group, 37.7 ± 0.1℃; firocoxib-3 group, 37.9 ± 0.2℃; p = 0.29).
Complications during the recovery period were recorded after 8 h after surgery. Cats in the control, firocoxib-1, and firocoxib-3 groups exhibited decreased appetite (37.5%, 25%, and 50%, respectively). The decrease in appetite in the control group did not differ significantly from those in the firocoxib-1 and firocoxib-3 groups (p = 0.59 and 0.61, respectively). One cat from the firocoxib-3 group vomited once at 48 h post-operation (12.5% of the group). No significant differences were found among the treatment groups for hemogram and serum biochemistry for hepatic injury (ALT and ALK) at baseline or at 72 h post-operation. Blood chemistry results for renal function (BUN and creatinine) were within normal limits in the control and firocoxib-1 group at baseline and at 72 h post-operation. There were 2 cats with reversible azotemia after surgery in the firocoxib-3 group, resulting in a significant increase in average BUN and creatinine level at 72 h post-operation over those at baseline (Table 3).
Firocoxib has been used successfully for postoperative pain control in dogs, but its effectiveness for pain control in cats has not been previously described. In the present study, we tested the postoperative analgesic effects of oral administration of firocoxib at 1 mg/kg and 3 mg/kg doses in cats after undergoing ovariohysterectomy. The results of the present study indicate that cats receiving 3 mg/kg firocoxib had lower postoperative pain scores (both Colorado pain scale scores and composite pain scores) than those in the control group. The firocoxib-3 group also had lower pain scores than cats receiving firocoxib-1, but the difference in scores between those groups was not statistically significant. The buccal bleeding times were comparable among the control, firocoxib-1, and firocoxib-3 groups. Side effects noted in the study included vomiting (one cat in the firocoxib-3 group) and azotemia (two cats in the firocoxib-3 group).
Monitoring the expression of postoperative pain in cats is challenging because both stress and pain can affect a cat's behavior [734]. In cats, the assessment of surgical wounds by touching may provide a better assessment of pain than that from observation [6]; therefore, visual analog scores were not used in the present study. Both Colorado pain scale scores and composite pain scores were chosen to detect pain expression in the cats [1]. Although comparisons of the specificities of the Colorado pain scale scores and composite pain scores in the cats were not evaluated in this study, the application of both of these monitoring methods is likely to increase sensitivity for pain detection in cats [6].
Confounding factors that may have affected the results of the present study include the use of preanesthetic drugs and variation in patient temperament. Various preanesthetic drugs provide strong additional analgesic properties and may interfere with an experiment. We chose isoflurane, which has a hypnosis analgesic effect, to reduce pain during anesthesia, whereas morphine was selected as a rescue analgesic drug for cats expressing moderate to severe pain [31]; the latter drug was not needed. Patient temperament also may have been a confounding factor because the evaluation of feline pain scores is partly based on temperament. Patients with severe anxiety probably would have higher pain scores than those who remain calm. Randomized control trials before surgery are important to reduce the confounding effect of behavior on pain assessment [3]. In the present study, since behavior may affect pain score measurement, cats with baseline composite pain scores greater than 8 (38% of the full composite pain scores) were considered aggressive and were excluded from the study. Moreover, there were no significant differences in the baseline Colorado pain scale and composite pain scores among the groups. In addition, cats that experienced pain with a composite pain scores greater than 12 (57% of the full composite pain scores) would have undergone a rescue analgesic procedure by administration of morphine and would have been removed from the study [1]. However, in this experiment, there were no cats requiring rescue analgesia during the recovery period. The reason for the absence of a need for rescue analgesia is probably due to an efficient neutering technique that uses a small incision (usually less than 2 cm long) performed by a highly experienced veterinarian via midline coeliotomy, a procedure that has been shown to be associated with fewer complications that those arising from flank laparotomy [9].
Firocoxib is classified as a COX-2 selective inhibitor and has been studied by various researchers as an analgesic medicine for post-operation pain control and for osteoarthritis management in dogs. There are a limited number of studies on firocoxib use in cats. One study evaluated the safety of using firocoxib in cats by administering a dosage of 0.75 to 3 mg/kg and indicated that firocoxib is effective for pyrexia control with minimal side effects [25]. It is well known that the safety margin for the use of NSAIDs in cats is relatively lower than that for dogs [1035]. In the present study, 1 or 3 mg/kg dosages of firocoxib were chosen, while the dose recommendation for dogs is 5 mg/kg for pain control. Administration of 3 mg/kg firocoxib significantly lowered the average Colorado pain scale scores and composite pain scores compared to the scores of the control group. However, although the cats that received 1 mg/kg firocoxib had lower Colorado pain scale scores and composite pain scores than those of the control group, the difference between the groups was not statistically significant. It is likely that 1 mg/kg firocoxib is not as effective for postoperative pain control as that from 3 mg/kg firocoxib. It should be noted that in all groups at three days post-operation, both the Colorado pain scale and composite pain scores had declined toward the baseline scores. Based on the present results, it is likely that ovariohysterectomy causes mild to moderate pain that lasts only for three days.
Some NSAIDs may interfere with blood clotting and may increase the risk of bleeding in patients undergoing surgery. The effect of firocoxib on bleeding disorders in cats has not been reported. Previously, it has been shown that a firocoxib dosage of 1 mg/kg was effective and inhibited production of PGE1 and PGE2 from the duodenal mucosal membrane [16]. Interestingly, at 1 mg/kg of firocoxib there was no effect on serum TXB2 level, which is important for platelet function [16]. In the present study, buccal mucosal bleeding times in the control group were compared with those in the firocoxib-1 or firocoxib-3 groups; there was no significant difference detected. The results suggest that the dosages of firocoxib used in the present study did not interfere with the pathway that controls platelet function and blood coagulation.
The effects of firocoxib administration on pyrexia control were also assessed in the present study. The body temperature of the cats was monitored during preoperative and postoperative periods, and no pyrexia was detected in any of the groups. Previous research indicates that firocoxib is a good pyrexia control medicine that protects against pyrogen-induced fever. In the present study, ovariohysterectomy induced only minimal inflammation and did not cause fever in the treatment groups. The role of firocoxib for fever control induced by inflammation may require further study.
The side effects of firocoxib in cats were also monitored in this study. There were two cats (25%) in the firocoxib-3 group with elevated creatinine and BUN levels compared to those in the preoperative period. Cats in the firocoxib-1 group did not show any obvious side effects. Some cats in the firocoxib-1 group had decreased appetite after surgery, but the incidence did not differ significantly from that in the control group. Vomiting was identified in one cat (12.5%) in the firocoxib-3 group at 48 h after surgery. However, because urinalysis was not recorded, we cannot determine whether the cause of vomiting was a renal or gastrointestinal side effect of firocoxib.
Blood pressure monitoring is essential for detection of hypotension that can occur during general anesthesia. Hypotension occurring during surgery can lead to insufficient blood flow to kidney, especially in cats with pre-existing disease [433]. The present study included healthy cats undergoing ovariohysterectomy and a preanesthetic blood check did not detect existing renal disease in any of the cats. It is essential for veterinary practitioners to increase the awareness that monitoring blood pressure during surgery can improve standard care for patients, especially for animals with pre-existing renal conditions [4]. Although the present study did not monitor blood pressure in all cats, intravenous fluid was given to all cats during the perioperative period. It should be noted that inhibition of cyclooxygenase enzyme by NSAIDs may lead to a significant reduction of renal blood flow due to the suppression of vasoactive prostaglandin synthesis. Two cats in the firocoxib-3 group with azotemia responded well to fluid therapy and the animals recovered without other complications.
Firocoxib has been shown to be highly effective and acceptable to control pain and inflammation due to osteoarthritis in canines. It also has been used effectively for postoperative pain control. In previous research, none of the healthy dogs presented clinical side effects; moreover, they had normal buccal mucosal bleeding times, no blood in their feces, and a normal gastrointestinal tract. In addition, no significant differences in blood profiles were detected between treatment and control groups [827]. However, researchers administrating firocoxib to treat osteoarthritis in geriatric dogs for 90 days reported diarrhea, vomiting, dark feces, and anorexia [19]. Cats with osteoarthritis may require a long-term use of analgesia. In the present study, firocoxib at a 1 mg/kg dosage was administered for 3 days without obvious side effects, and the 3 mg/kg firocoxib dose did produce gastrointestinal and renal side effects. Regardless, the application and dosage of firocoxib for long-term use in cats warrants further study.
In conclusion, the present study provides evidence that the selective COX-2 drug firocoxib is useful for postoperative pain control in cats. A firocoxib dose of 3 mg/kg provided better postoperative pain control than that from a firocoxib dose of 1 mg/kg; however, the gastrointestinal and renal function side effects detected at the higher dose of firocoxib might outweigh the pain control benefit. Based on our results, short-term use of the lower dose of firocoxib (1 mg/kg) is recommended for cats.
Figures and Tables
Fig. 2
Colorado pain scale scores of control, firocoxib-1, and firocoxib-3 groups. Differences were not significant among control, firocoxib-1, and firocoxib-3 groups.

Fig. 3
Average Colorado pain scale scores over the 2 to 48 h post-operation of control, firocoxib-1, and firocoxib-3 groups. *p < 0.05 vs. control.
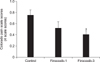
Fig. 4
Composite pain scores in cats after ovariohysterectomy at 0 (baseline), 2, 5, 8, 24, 30, and 48 h post-operation. *p < 0.05 control vs. firocoxib-3 at 24 h post-operation.

Table 1
Colorado pain scale scores* (0–4 scores) for feline patients

*Colorado pain scale score criteria were modified from previous study [12].
Table 2
Composite pain scores* for feline patients

*Composite pain score criteria were modified from previous study [6].
Acknowledgments
The authors thank the study's surgeon, Dr. Darut Kingkand, and staffs at Praholyothin 48 Small Animal Hospital for their efforts during the study, including the provision of animal care. Also, the authors thank Kasetsart University Veterinary Teaching Hospital, Bangkhen campus, for providing technical support. This study was supported by grants from the National Research Council of Thailand and the Kasetsart University Research and Development Institute (KURDI).
References
1. Al-Gizawiy MM, Rudé EP. Comparison of preoperative carprofen and postoperative butorphanol as postsurgical analgesics in cats undergoing ovariohysterectomy. Vet Anaesth Analg. 2004; 31:164–174.

2. Anderson KE, Austin J, Escobar EP, Carbone L. Platelet aggregation in rhesus macaques (Macaca mulatta) in response to short-term meloxicam administration. J Am Assoc Lab Anim Sci. 2013; 52:590–594.
3. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46:399–424.

4. Bednarski R, Grimm K, Harvey R, Lukasik VM, Penn WS, Sargent B, Spelts K, American Animal Hospital A. AAHA anesthesia guidelines for dogs and cats. J Am Anim Hosp Assoc. 2011; 47:377–385.

5. Blois SL, Allen DG, Wood RD, Conlon PD. Effects of aspirin, carprofen, deracoxib, and meloxicam on platelet function and systemic prostaglandin concentrations in healthy dogs. Am J Vet Res. 2010; 71:349–358.

6. Brondani JT, Luna SPL, Padovani CR. Refinement and initial validation of a multidimensional composite scale for use in assessing acute postoperative pain in cats. Am J Vet Res. 2011; 72:174–183.

7. Calvo G, Holden E, Reid J, Scott EM, Firth A, Bell A, Robertson S, Nolan AM. Development of a behaviour-based measurement tool with defined intervention level for assessing acute pain in cats. J Small Anim Pract. 2014; 55:622–629.

8. Camargo JB, Steagall PVM, Minto BW, de Sá Lorena SER, Mori ES, Luna SPL. Post-operative analgesic effects of butorphanol or firocoxib administered to dogs undergoing elective ovariohysterectomy. Vet Anaesth Analg. 2011; 38:252–259.

9. Coe RJ, Grint NJ, Tivers MS, Hotston Moore A, Holt PE. Comparison of flank and midline approaches to the ovariohysterectomy of cats. Vet Rec. 2006; 159:309–313.

10. Court MH, Greenblatt DJ. Molecular basis for deficient acetaminophen glucuronidation in cats. An interspecies comparison of enzyme kinetics in liver microsomes. Biochem Pharmacol. 1997; 53:1041–1047.
11. Curry SL, Cogar SM, Cook JL. Nonsteroidal antiinflammatory drugs: a review. J Am Anim Hosp Assoc. 2005; 41:298–309.

12. Epstein ME, Rodan I, Griffenhagen G, Kadrlik J, Petty MC, Robertson SA, Simpson W. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg. 2015; 17:251–272.

13. Fenster MN. Nitrous oxide as an inhalation anesthetic. J Med Soc N J. 1951; 48:281.
14. Fu JY, Masferrer JL, Seibert K, Raz A, Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990; 265:16737–16740.

15. Goodman L, Torres B, Punke J, Reynolds L, Speas A, Ellis A, Budsberg S. Effects of firocoxib and tepoxalin on healing in a canine gastric mucosal injury model. J Vet Intern Med. 2009; 23:56–62.

16. Goodman LA, Torres BT, Reynolds LR, Budsberg SC. Effects of firocoxib, meloxicam, and tepoxalin administration on eicosanoid production in target tissues of healthy cats. Am J Vet Res. 2010; 71:1067–1073.

17. Hanson PD, Brooks KC, Case J, Conzemius M, Gordon W, Schuessler J, Shelley B, Sifferman R, Drag M, Alva R, Bell L, Romano D, Fleishman C. Efficacy and safety of firocoxib in the management of canine osteoarthritis under field conditions. Vet Ther. 2006; 7:127–140.
18. Hewson CJ, Dohoo IR, Lemke KA. Perioperative use of analgesics in dogs and cats by Canadian veterinarians in 2001. Can Vet J. 2006; 47:352–359.
19. Joubert KE. The effects of firocoxib (Previcox) in geriatric dogs over a period of 90 days. J S Afr Vet Assoc. 2009; 80:179–184.

20. Kondo Y, Takashima K, Matsumoto S, Shiba M, Otsuki T, Kinoshita G, Rosentel J, Gross SJ, Fleishman C, Yamane Y. Efficacy and safety of firocoxib for the treatment of pain associated with soft tissue surgery in dogs under field conditions in Japan. J Vet Med Sci. 2012; 74:1283–1289.

21. Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991; 266:12866–12872.

22. Lascelles BDX, Court MH, Hardie EM, Robertson SA. Nonsteroidal anti-inflammatory drugs in cats: a review. Vet Anaesth Analg. 2007; 34:228–250.

23. Lecoindre O, Pepin-Richard C. Tolerance of firocoxib in dogs with osteoarthritis during 90 days. J Vet Pharmacol Ther. 2011; 34:190–192.
24. Mathews KA, Pettifer G, Foster R, McDonell W. Safety and efficacy of preoperative administration of meloxicam, compared with that of ketoprofen and butorphanol in dogs undergoing abdominal surgery. Am J Vet Res. 2001; 62:882–888.

25. McCann ME, Rickes EL, Hora DF, Cunningham PK, Zhang D, Brideau C, Black WC, Hickey GJ. In vitro effects and in vivo efficacy of a novel cyclooxygenase-2 inhibitor in cats with lipopolysaccharide-induced pyrexia. Am J Vet Res. 2005; 66:1278–1284.

26. Mellor PJ, Roulois AJA, Day MJ, Blacklaws BA, Knivett SJ, Herrtage ME. Neutrophilic dermatitis and immune-mediated haematological disorders in a dog: suspected adverse reaction to carprofen. J Small Anim Pract. 2005; 46:237–242.

27. Monteiro-Steagall BP, Steagall PVM, Lascelles BDX. Systematic review of nonsteroidal anti-inflammatory druginduced adverse effects in dogs. J Vet Intern Med. 2013; 27:1011–1019.

28. Orsini JA, Ryan WG, Carithers DS, Boston RC. Evaluation of oral administration of firocoxib for the management of musculoskeletal pain and lameness associated with osteoarthritis in horses. Am J Vet Res. 2012; 73:664–671.

29. Peskar BM. Role of cyclooxygenase isoforms in gastric mucosal defence. J Physiol Paris. 2001; 95:3–9.

30. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011; 31:986–1000.

31. Sanders RD, Patel N, Hossain M, Ma D, Maze M. Isoflurane exerts antinociceptive and hypnotic properties at all ages in Fischer rats. Br J Anaesth. 2005; 95:393–399.

32. Soll AH, McCarthy D. NSAID-related gastrointestinal complications. Clin Cornerstone. 1999; 1:42–56.

33. Sparkes AH, Heiene R, Lascelles BDX, Malik R, Sampietro LR, Robertson S, Scherk M, Taylor P. ISFM and AAFP consensus guidelines: long-term use of NSAIDs in cats. J Feline Med Surg. 2010; 12:521–538.

34. Väisänen MAM, Tuomikoski SK, Vainio OM. Behavioral alterations and severity of pain in cats recovering at home following elective ovariohysterectomy or castration. J Am Vet Med Assoc. 2007; 231:236–242.

35. Villar D, Buck WB, Gonzalez JM. Ibuprofen, aspirin and acetaminophen toxicosis and treatment in dogs and cats. Vet Hum Toxicol. 1998; 40:156–162.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download