Abstract
To determine heat-shock protein (Hsp)90 expression is connected with cellular apoptotic response to heat stress and its mechanism, chicken (Gallus gallus) primary myocardial cells were treated with the Hsp90 promoter, aspirin, and its inhibitor, geldanamycin (GA), before heat stress. Cellular viability, heat-stressed apoptosis and reactive oxygen species level under different treatments were measured, and the expression of key proteins of the signaling pathway related to Hsp90 and their colocalization with Hsp90 were detected. The results showed that aspirin treatment increased the expression of protein kinase B (Akt), the signal transducer and activator of transcription (STAT)-3 and p-IKKα/β and the colocalization of Akt and STAT-3 with Hsp90 during heat stress, which was accompanied by improved viability and low apoptosis. GA significantly inhibited Akt expression and p-IKKα/β level, but not STAT-3 quantity, while the colocalization of Akt and STAT-3 with Hsp90 was weakened, followed by lower cell viability and higher apoptosis. Aspirin after GA treatment partially improved the stress response and apoptosis rate of tested cells caused by the recovery of Akt expression and colocalization, rather than the level of STAT-3 (including its co-localization with Hsp90) and p-IKKα/β. Therefore, Hsp90 expression has a positive effect on cellular capacity to resist heat-stressed injury and apoptosis. Moreover, inhibition of Hsp90 before stress partially attenuated its positive effects.
Organisms and cells are constantly exposed to a variety of stressors such as heat, heavy metals, and anoxia, among which the worst environmental condition affecting poultry is heat stress. Heat stress can result in great economic losses by reducing growth rate and hatchability and increasing mortality [29]. Previous studies have shown that heat stress could cause a cellular imbalance between the production of reactive oxygen species (ROS) and antioxidant systems, then further stimulate the ROS production [10], accompanied by oxidative damage [27]. Reactive oxygen metabolites trigger a self-protective mechanism to resist stress known as the heat stress response, which involves the synthesis of heat-shock proteins (Hsps) to protect cells from oxidative stress injuries or repair injured cells/tissues [16].
Some reports have demonstrated a strong relationship between oxidation and Hsp70 expression [15]. Like Hsp70, Hsp90 is an abundant molecular chaperone involved in cytoprotection. Inducible Hsp90 folds, stabilizes and functionally regulates a variety of cellular proteins in response to stress insults [7]. Hsp90 also helps stabilize and mature the conformation of “client” proteins, which include key mediators of signal transduction in cell survival [11]. However, studies of the protective effects of Hsp90 expression against oxidative damage of myocardial cells induced by heat stress are rare. Controlling the expression or function of Hsp90 experimentally provides a method to investigate the effect of Hsp90 on heat stress injures of myocardial cells systematically. Previous studies showed that long-term administration of aspirin increased Hsp expression [20]. Moreover, co-exposure of the fish cell, CHSE-214, to aspirin and heat shock (24℃) enhanced heat shock-induced Hsp70 expression via activation of heat shock factors [23]. Our previous in vivo studies revealed that aspirin could induce Hsp90 overexpression and the subsequent activation of protein kinase B (Akt). Therefore, this study was conducted to verify aspirin as a stimulant of Hsp90 overexpression in vitro. Geldanamycin (GA) is a naturally as amycin antibiotic that is a specific inhibitor of Hsp90. GA functions by binding to Hsp90, preventing chaperone-client binding [1628]. Inhibition of Hsp90 by GA was recently found to effectively prevent IκB kinase (IKK) biosynthesis and subsequent IKK and nuclear factor-kappa (NF-κB) binding activation in cells. Therefore, we selected GA as the Hsp90 inhibitor for this study [3].
Cells have developed complicated mechanisms, in addition to the Hsps induction, to respond to excess ROS during oxidative stress. Cells also respond to oxidative stress by activating multiple signal transduction pathways [29]. The signal transducer and activator of transcription (STAT) pathway is an important signaling mechanism that transduces the signal carried by extracellular polypeptides to the cell nucleus, where activated STAT proteins modify gene expression [29]. Akt, which is activated by various receptors for growth factors, cytokines and other signaling molecules, is well characterized in regulating cell apoptosis or survival [13]. The NF-κB pathway is also involved in the cellular response to stress, as well as in controlling the transcription of DNA. NF-κB activation response to stimuli results from phosphorylation and proteolytic degradation of IκB by IKK [9]. STAT, Akt and IKK are all client proteins of Hsp90 that play important roles in the stress response. Moreover, upregulating Hsp90 is an environmentally adaptive strategy to resist stress through maintenance of protein conformation and activation of the signaling pathways of key proteins [2].
The objective of the present study was to more fully understand the effects of Hsp90 expression on the response to oxidative stress of chicken myocardial cells exposed to heat stress in vitro using the promoter aspirin and inhibitor GA.
Thiazolyl blue and aspirin were purchased from Genview and Sigma (USA), respectively. GA was purchased from Dingsibio (China). Anti-Hsp90 antibodies were purchased from Enzo (USA), anti-Akt antibodies were purchased from Cell Signaling Technology (USA), anti-phospho-IKKα/β and anti-STAT-3 antibodies were obtained from Santa Cruz Biotechnology (USA), anti-heat shock factor 1 (HSF-1) antibodies were obtained from Abcam (USA), and anti-GAPDH antibodies were obtained from EarthOx Life Sciences (USA). The ROS assay kit, FITC annexin V apoptosis detection kit and caspase-3, 8 and 9 assay kits were obtained from Beyotime Biotechnology (China), BD Pharmingen (USA), and AOGENE (China), respectively. The bicinchoninic acid (BCA) protein quantitative assay kit was purchased from Dingguo (China).
Chicken primary myocardial cells isolated from the hearts of 11-day-old chicken embryos were provided for experimental use by Applied Biological Materials (Canada). Cells were cultivated in cell culture plates containing Dulbecco's modified Eagle's medium with high glucose supplemented with 20% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin for 48 h at 37℃ in a CO2 incubator to ensure that a minimum of 90% of the cells in the culture plates were alive.
Chicken primary myocardial cells were treated with heat stress at 42℃ for 0, 1, 2, 3, 5 and 7 h. The heat-stressed cells were used to detect the cell viability by MTT assay, the apoptosis rate by annexin V and propidium iodide via flow cytometry, and the ROS level by measuring the absorbance of 2′,7′-dichlorodihydrofluorescein (DCF) fluorescence with fluorescence microplate reader (FLx800; BioTek, USA).
Chicken primary myocardial cells were treated with: (1) 0, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5 and 1 mg/mL aspirin for 24 h, after which their viability was measured; (2) 0, 0.01, 0.1 and 1 mg/mL aspirin for 2 h and evaluated for Hsp90 expression by Western blot; and (3) 1 mg/mL aspirin for 0 h, 30 min, 1 h, 1.5 h, 2 h, 4 h and 8 h and probed for the Hsp90 expression by Western blot.
Chicken primary myocardial cells were treated with 0, 0.01, 0.05, 0.1, 0.5, 1, 5 and 10 µM GA for 24 h, after which their viability was measured. Chicken primary myocardial cells were pre-treated with 10 µM GA, 1 µM GA, 0.1 µM GA and 0 µM GA for 14 h, then treated with 1 mg/mL aspirin or not for 2 h, after which the Hsp70 and Hsp90 expression was measured by Western blot.
Chicken primary myocardial cells were pre-treated with 0 or 0.1 µM GA for 14 h, then treated with 1 mg/mL aspirin or not for 2 h, after which the location of HSF-1 was detected by immunofluorescence microscopy.
Chicken primary myocardial cells were pre-treated with 0 µM GA or 0.1 µM GA for 14 h and then treated with 1 mg/mL aspirin or not for 2 h, after which they were heat-stressed at 42℃ for 5 h. The expression of the aforementioned proteins in the treated cells were analyzed by Western blotting, while they were analyzed for co-localization by immunocytochemistry, and for caspases activities by enzyme-linked immunosorbent assay (ELISA).
The supernatant of treated cells was removed, and 0.9 mL medium and 0.1 mL MTT solution (5 mg/mL) were added to the cell plates. Incubation was conducted at 37℃ in the CO2 incubator for 3 h, after which 1 mL of dimethyl sulfoxide was added and the absorbance at 490 nm was measured.
Treated cells were washed with pre-cooled PBS, then digested with trypsin for 30 min at 37℃. The medium containing serum was added to terminate the digestion. Next, the cell suspension was centrifuged (1,000 × g) for 5 min, after which the sediment was washed with pre-cooled PBS twice. Binding buffer (300 µL) was subsequently added for detection, which was conducted according to the manufacturer's instructions.
The determination of cellular ROS levels was conducted according to the manufacturer's instructions. The DCFH-DA (2,7-dichlorodihydrofluorescein diacetate) probe was loaded into cells before the test. After the appropriate processing, cells were digested with trypsin for 5 min at 37℃. The cell suspension was then collected for detection of the absorbance of DCF fluorescence.
Cells were washed with ice-cold PBS, then lysed with a protein extraction reagent containing phenylmethylsulfonyl fluoride. Cell lysates were centrifuged at 13,000 × g for 5 min at 4℃ to remove cellular debris, after which the supernatants were collected. The total protein concentration was measured using a BCA protein quantitative analysis kit. The total protein samples were stored at −20℃ for protein quantification.
Cell protein samples (40 µg) were separated on an SDS-polyacrylamide gel and electrotransferred to polyvinylidene fluoride membranes. The membranes were then incubated overnight with primary antibodies at 4℃, washed with Tris-buffered saline with Tween 20 and further incubated with horse radish peroxidase-conjugated second antibodies at room temperature for 2 h. Antibody-antigen complexes were detected using Western blotting luminol reagent (Bio-Rad Laboratories, USA). Finally, reactive bands were quantified using the Quantity One software (ver. 4.6.2; Bio-Rad Laboratories). GADPH was used as the loading control.
Treated myocardial cells were fixed with 4% paraformaldehyde, then blocked with 5% BSA for 30 min after incubation with 0.5% Triton X-100 and washing with PBS. The cells were incubated with primary antibodies for HSF-1, Akt plus Hsp90 and STAT-3 plus Hsp90 at 37℃ for 1 h, then incubated with rhodamine (plus fluorescein isothiocyanate)-conjugated secondary antibodies at 37℃ for 1 h. After washing with PBS, cell nuclei were stained with DAPI (4,6-diamidino-2-phenylindole). Photographs of cells were taken under a fluorescence microscope.
SPSS Statistics for windows (ver. 20.0; IBM, USA) was used for all statistical analyses by one-way analysis of variance followed by the least significant difference multiple comparison test. The Student's t-test was used to compare the variables between two groups. Results were expressed as the means ± SD. All experiments were performed in triplicate (n = 3).
To determine the optimal heat stress time for subsequent tests, we measured the influence of heat stress on cellular viability, apoptosis and oxidative stress levels of chicken myocardial cells (Fig. 1). We found that, from 1 h of heat stress, the cellular viability decreased (p < 0.01), while the apoptosis rate increased (p < 0.01), accompanied by a rise ROS levels. However, the apoptosis rate of myocardial cells at 5 h was significantly higher (p < 0.01) than that from 1 h to 3 h. Therefore, we selected 5 h of heat stress for the subsequent tests.
To determine the optimum concentration and treatment time for aspirin, cellular viability and Hsp90 expression were measured after aspirin treatment (Fig. 2). None of the concentrations of aspirin used affected the viability of chicken myocardial cells. The Hsp90 expression of myocardial cells increased significantly in response to aspirin at concentrations of 0.1 and 1 mg/mL after 2 h of treatment. Chicken myocardial cells could significantly upregulate (p < 0.01) the Hsp90 expression from 1 h of aspirin administration at 1 mg/mL. Therefore, treatment with 1 mg/mL aspirin for 2 h was selected for subsequent tests.
To verify the inhibitory effect of GA on Hsp90, we measured Hsp70 and Hsp90 expression, as well as the translocation of HSF-1 (Figs. 3 and 4). The results showed that when chicken primary myocardial cells were treated with GA at 0.01 to 10 µM, cell viability was not affected. At these GA concentrations, the Hsp90 expression level decreased, which was accompanied by elevated Hsp70 expression. Additionally, cells treated with GA showed higher nuclear localization of HSF-1 than non-treated cells and those treated with aspirin. When the cells were treated with GA (0.1 µM GA) for 14 h followed by aspirin treatment for 2 h, nuclear translocation of HSF-1 was also detected. Thus, treatment with GA at 0.1 µM for 14 h was selected for the subsequent tests.
The effects of different treatments on Hsp90 expression are shown in Fig. 5. Aspirin treatment increased Hsp90 expression (p < 0.05) in myocardial cells exposed to heat stress when compared with cells treated by heat stress alone, while GA treatment decreased the Hsp90 level significantly. However, aspirin treatment did not effectively stimulate the Hsp90 expression of myocardial cells exposed to GA in advance.
As shown in Fig. 6, both aspirin treatment and co-treatment with GA and aspirin increased (p < 0.01) Akt expression. GA treatment decreased the Akt level to some extent compared with that in cells treated only by heat stress. Moreover, the Akt level in myocardial cells co-treated with GA and aspirin was significantly higher than in those treated with GA alone, and very close to the level in cells treated with aspirin alone. In addition, when compared with cells treated only by heat stress, STAT-3 expression increased significantly in all treatments (aspirin, GA, and GA and aspirin). Moreover, the STAT-3 levels in response to both aspirin treatment and GA treatment were higher (p < 0.01) than that in response to co-treatment with GA and aspirin.
Upper panels in Fig. 7 shows the co-localization results of Akt and Hsp90 in chicken primary myocardial cells exposed to heat stress. In non-treated cells, Akt was co-localized with Hsp90 in the cytoplasm of myocardial cells (panel A1 in Fig. 7). After exposure to heat stress, double immunofluorescent staining in the cytoplasm was slightly enhanced and co-localization of Akt and Hsp90 was observed in the nucleus (panel A2 in Fig. 7). Aspirin treatment significantly increased the signal intensity of co-localization of Akt and Hsp90 in the both cytoplasm and the nucleus (panel A3 in Fig. 7). GA treatment reduced the co-localization staining of Akt and Hsp90 compared with that in non-treated cells (panel A4 in Fig. 7). When cells were co-treated with GA and aspirin, the co-localization signal of Akt and Hsp90 in the cytoplasm and nucleus were increased relative to that in cells treated with GA alone (panel A5 in Fig. 7).
Bottom panels in Fig. 7 shows the co-localization results of STAT-3 and Hsp90 in chicken primary myocardial cells exposed to heat stress. In the non-treated cells, co-localization of STAT-3 and Hsp90 was very rare, and a weak STAT-3 signal was observed in the nucleus (panel B1 in Fig. 7). Heat stress enhanced the co-localization of STAT-3 and Hsp90, as well as the nuclear translocation of SATAT-3 (panel B2 in Fig. 7). Aspirin treatment strengthened the signal intensity of co-localization of STAT-3 and Hsp90 and its nuclear translocation significantly (panel B3 in Fig. 7). When the cells were treated with GA, the co-localization of STAT-3 and Hsp90 and the nuclear signal of STAT-3 disappeared completely (panel B4 in Fig. 7). When myocardial cells were first treated with GA, subsequent aspirin treatment reinstated the co-localization of STAT-3 and Hsp90 and its nuclear translocation, to some extent (panel B5 in Fig. 7).
The p-IKKα/β level in chicken primary myocardial cells is shown in Fig. 8. Following heat stress, aspirin upregulated the p-IKKα/β level (p < 0.05), while GA treatment and co-treatment with GA and aspirin downregulated the p-IKKα/β level significantly (p < 0.05 and p < 0.01, respectively) relative to cells treated by heat stress alone. The p-IKKα/β level in myocardial cells co-treated with GA and aspirin was significantly lower (p < 0.01) than that in cells treated with aspirin or GA alone.
The effect of different treatment on chicken primary myocardial cells exposed to heat stress is shown in Fig. 9. After exposure to heat stress, aspirin treatment increased the viability of myocardial cells (p < 0.05), which was accompanied by a significantly lower apoptosis rate (p < 0.01) and reduced ROS level (p < 0.01) compared with those treated by heat stress alone. Moreover, GA treatment decreased the viability of myocardial cells (p < 0.05), which was accompanied by a higher apoptosis rate and increased ROS levels (p < 0.05) when compared to those treated by heat stress only. When chicken myocardial cells treated with GA first and then with aspirin were heat stressed, the apoptosis rate and ROS levels were lower (p < 0.05) relative to those treated by GA only, while cell viability was lower (p < 0.01) than those treated by aspirin only, and there was a higher apoptosis rate (p < 0.05) and ROS level (p < 0.01).
The caspase-3, 8 and 9 activities in chicken primary myocardial cells exposed to heat stress are shown in Fig. 10. When compared with those treated by heat stress alone, aspirin treatment induced a significant (p < 0.05) upregulation of caspase-3 and downregulation (p < 0.01) of caspase-8 and caspase-9. GA treatment increased the activity of caspase-3 significantly, while co-treatment with GA and aspirin increased the activities of caspase-3 (p < 0.01) and caspase-9 (p < 0.05). The activities of caspase-3 and caspase-9 in the myocardial cells treated first by GA and then by aspirin were higher (p < 0.01) than in those treated by aspirin and lower (p < 0.01) than in those treated by GA. The activity of caspase-8 in myocardial cells co-treated with GA and aspirin was higher (p < 0.01) than in those treated by aspirin and lower (p < 0.05) than in those treated by GA.
Previous studies reported that heat stress could cause severe damage to myocardial cells in rats, accompanied by an increase in apoptotic cells [19]. Heat stress would also induce superfluous ROS production and oxidative damage to cellular proteins and DNA [1627], which was consistent with our results. The present study showed that from 1 h of heat stress, chicken primary myocardial cells were seriously injured, as indicated by reduced cell viability, increased cell apoptosis and intracellular ROS levels. The stress damage was more significant after 5 h of heat stress, especially in terms of apoptosis, suggesting that heat stress could induce oxidative stress and related cell apoptosis in vitro, which was in line with the results of Islam et al. [19] in heat-stressed rat primary myocardial cells. There was a strong negative correlation between Hsp90 expression and ROS level/cell apoptosis; namely, high expression of Hsp90 increased anti-stress capacity [1619].
Aspirin, which is used to reduce fever, lowers the temperature threshold needed to induce HSPs and promotes thermotolerance [114]. Our previous in vivo study showed that aspirin administration could increase Hsp90 expression in chicken myocardial cells with or without heat stress (data not shown). In the present study, aspirin treatment for 1 h or longer stimulated Hsp90 expression without affecting cell viability. Translated HSF-1 or purified HSF in vitro could be activated by low pH (pH 6.5). Aspirin is an organic acid; therefore, its effects on HSF activity could be similar to the activating effects of low pH on HSF-1 conformation [38]. Aspirin causes HSF-1 to be phosphorylated and form an HSF-1-HSE complex, which is necessary to activate transcription [21].
GA competes with ATP for binding to the ATP-binding pocket of Hsp90, then inhibits the chaperone function of Hsp90, and reduced Hsp90 expression was also observed after GA treatment [6]. In addition, HSF-1 could translocate from the cytosol to the nucleus in response to Hsp90 inhibition [34]: when Hsp90 is not bound to a client protein, it binds to HSF-1 and other co-chaperone proteins, after which HSF-1 is activated and released from the co-chaperone complex. Activated HSF-1 translocates to the nucleus where it stimulates the transcription of Hsp70 gene [4]. Previous studies also showed that Hsp70 expression was increased when Hsp90 was inhibited, which was often used to confirm Hsp90 inhibition [36]. Hsp90 plays a central role in the conformational maturation of Hsp90-associated client proteins. GA-induced degradation of Hsp90 client proteins can result in decreased Hsp90 levels [6]. In this study, GA at 0.1 µM or above significantly inhibited Hsp90 expression in myocardial cells and induced Hsp70, while 0.1 µM GA induced the nuclear translocation of HSF-1. Taken together, these results illustrated that GA has a sufficient inhibitory effect on Hsp90 expression in chicken myocardial cells.
When exposed to heat and/or oxidation stress, HSPs, especially Hsp70 and Hsp90, could maintain cell functions and metabolism to enhance their resistance to stress damage, thereby increasing cell survival rate [1516]. Hsp90 level is positively correlated with cellular survival and antioxidant capacity [1637]. As expected, because of the self-protective consumption of heat-stressed myocardial cells, the Hsp90 level was obviously decreased. At that time, Hsp90 participates in cellular cytoprotection through folding, stabilizing, and functionally regulating cellular proteins in response to stimulation [7]. Cells also respond to oxidant injury through the activation of multiple signal transduction pathways, of which many key mediators are client proteins of Hsp90 whose conformational maturation and/or stability requires Hsp90 [1129]. According to our results, heat stress resulted in a significant decrease in cell viability and a significant increase in cell apoptosis and ROS levels, illustrating that the Hsp90 consumption exceed the cellular tolerance to heat stress. Our results also showed that aspirin treatment increased Hsp90 expression during heat stress, and that this was accompanied by lower oxidative stress levels and cellular apoptosis. Additionally, GA inhibited the Hsp90 synthesis, which was followed by lower viability and higher apoptosis rate/ROS levels, further suggesting that the Hsp90 level is particularly important to the anti-stress capacity of myocardial cells.
Increased Hsp90 provided tolerance against stress and controlled cell survival via its effect on Akt expression [30], which influences cell functions via several mechanisms involved in protein phosphorylation, apoptosis and cell proliferation [35]. Akt, a client of Hsp90, binds to the middle domain of Hsp90 [31]. Hsp90 inhibition increases Akt degradation, which results in decreased Akt abundance accompanied by reduction of Akt activation [30]. Hsp90 also interacts with STAT-1α and STAT-3, which are required for STAT activation characterized by its phosphorylation and translocation from the cytosol to the nucleus in stressed cells [33]. Furthermore, the results of the current study demonstrated a positive correlation between Hsp90 level and STAT activation [32], suggesting that Hsp90 was required for STAT activation. We found that pre-treatment with aspirin increased total Akt and STAT-3 expression in heat-stressed cells, and that GA treatment reduced cellular Akt expression rather than STAT-3. The positive correlation between Hsp90 level and Akt expression further verified the importance of Hsp90 on its client proteins. However, Hsp90 expression in cells under aspirin or GA treatment did not influence the total STAT-3 level significantly, which is consistent with the results of a previous study [32] that showed that STAT phosphorylation changed rather than total protein levels with fluctuating Hsp90 expression. Moreover, the co-localization of Hsp90 and two client proteins in this study showed that heat stress could induce co-localization to different degrees, which was enhanced significantly by aspirin pre-treatment and inhibited by GA pre-treatment. The co-localization level was also affected by Hsp90 expression, especially the nuclear translocation accompanying co-localization, which was positively related to the degree of cellular stress injury in this study. Therefore, we believe that Hsp90 affects the capacity of cellular anti-stress reactions directly by influencing the expression levels and/or co-localization status of its client proteins.
The NF-κB pathway is also essential for cells during stress responses [17]. The phosphorylation degradation of IκB by the IKK complex causes activation of NF-κB [3]. IKK is also an Hsp90 client protein, and the enhanced affinity of Hsp90 for IKK increases NF-κB activation [26]. Moreover, Hsp90 inhibition by GA inhibited Hsp90-IKK complex formation, resulting in an increase in IKKα/β degradation [24]. Taken together, these findings suggest that Hsp90 is required for IKK to activate NF-κB. Hsp90 is required for both IKK stabilization and activation through their physical interaction [3]. Our results support the role of Hsp90 to activate IKK. We found that the increase in Hsp90 level by aspirin was accompanied by a higher level of p-IKKα/β, while the inhibition of Hsp90 by GA was followed by lower expression of p-IKKα/β.
Our investigation of enzymes related to cell apoptosis included caspase-3, 8 and 9. Two apoptosis pathways have been recognized, the extrinsic death receptor pathway (activated by caspase-8) and the intrinsic mitochondrial pathway (controlled by caspase-9), which finally activates caspase-3, resulting in the cleavage of various proteins and causing cell apoptosis [51325]. The activities of all tested enzymes were influenced by heat stress to some extent, illustrating that apoptosis of chicken myocardial cells maybe related to both apoptosis pathways. Aspirin treatment effectively attenuated the activation of tested caspases through the induction of Hsp90, while inhibition of Hsp90 by GA activated the tested caspases, suggesting that Hsp90 expression was negatively correlated with cellular apoptosis.
According to our results, when Hsp90 expression was inhibited by GA, aspirin treatment could again increase Hsp90 expression, despite the rise being insignificant compared with that in cells treated by GA alone, which was accompanied by significant remission of the apoptosis rate and oxidant stress level. We believe the recovery of Akt expression accompanying the increased Hsp90 level might play an important role in the anti-oxidant stress and anti-apoptosis capabilities of cells. Akt forms a complex with Hsp90 that is necessary for Akt to perform various processes in cellular signaling [12]. When Akt is downregulated following inhibition of Hsp90 with GA, Akt-dependent survival activities are suppressed [8]. In the present study, aspirin treatment after treatment with GA stimulated Akt expression, enhanced its colocalization with Hsp90 and promoted the nuclear translocation of the complex in myocardial cells again. The direct correlation between resistance to oxidative stress and the Akt pathway has also been proposed by Ikeyama et al. [18]. Furthermore, Bcl-2 can inhibit apoptosis when stimulated by oxidative stress, and is primarily regulated by Akt [22]. Active Akt in association with Hsp90 is also reported to inhibit the activity of pro-apoptotic kinase ASK1. Hsp90-Akt binds to and phosphorylates ASK1 to maintain ASK1 in an inactive state [37]. These interactions contribute to the cellular pro-survival capacity related to Akt.
We also found that aspirin treatment after GA administration further decreased the total STAT-3 protein level. As stated above, Hsp90 is required for STAT phosphorylation. Therefore, the increase in Hsp90 expression induced by aspirin following GA would effectively promote the phosphorylation of STAT-3 resulting in STAT-3 levels decreasing significantly. Moreover, the co-localization of STAT-3 and Hsp90 did not increase significantly, which would also result in the degradation of STAT-3 protein considering the role of Hsp90 in conformation protection and structure stability [11]. Janus kinase 1/2 (JAK1/2), the upstream activator of STAT, is also an important client protein of Hsp90 [33]. Hence, JAK1/2 might be involved in regulation of STAT-3 level through its interaction with Hsp90. NF-κB is also a mitogen-activated protein kinase-regulated transcription factor that is activated by oxidative stress through the phosphorylation of its upstream factor, IKK [29]. The present study demonstrated that aspirin treatment after GA further decreased the p-IKKα/β level relative to that in cells treated with GA alone, which might reflect a reinforced interaction between Hsp90 and its client protein, IKK, while Hsp90 induction by aspirin led to a greater restriction in the phosphorylation level of IKK itself, leading to reduced stress-related cell damage.
In conclusion, Hsp90 expression has a positive effect on the cell's capacity to resist heat stress injury and avoid apoptosis, which was closely related to Akt expression, STAT activation through its phosphorylation, and IKK phosphorylation. However, when Hsp90 expression was inhibited before heat stress, the protective effect of subsequent Hsp90 induction was restricted to some extent. Therefore, to effectively resist heat-stress injury in chickens, preventive action such as better induction of Hsp90 through aspirin administration in advance is recommended.
Figures and Tables
 | Fig. 1Effect of heat stress on chicken primary myocardial cells. (A) The effect of heat stress (HS) on cell viability. (B) The effect of HS on cell apoptosis rate. (C) The effect of HS on cellular reactive oxygen species (ROS) level. The difference of the data at different times vs. that at 0 h is indicated by *p < 0.05 and **p < 0.01. The difference of the data at 1 h, 2 h, 3 h vs. that at 5 h and the data at 1 h, 2 h, 3 h vs. that at 7 h are indicated by ††p < 0.01. OD, optical density. |
 | Fig. 2The induction of Hsp90 by aspirin (acetylsalicylic acid [ASA]) in chicken primary myocardial cells. (A) The effect of ASA on cell viability. (B) The effect of ASA at different concentrations on Hsp90 level of chicken primary myocardial cells. (C) The effect of ASA at 1 mg/mL on Hsp90 level of chicken primary myocardial cells. (A and B) The difference of data at different concentrations vs. that at 0 mg/mL is indicated by *p < 0.05 and by **p < 0.01. (C) The difference of the data at different times vs. that at 0 h is indicated by *p < 0.05 and by **p < 0.01. OD, optical density. |
 | Fig. 3Inhibitory effect of geldanamycin (GA) on Hsp90 in chicken primary myocardial cells. (A) The effect of GA on cell viability. (B) The effect of GA on Hsp70 and Hsp90 in chicken primary myocardial cells. The difference of the data of cells with different treatments vs. that of untreated cells is indicated by *p < 0.05 and by **p < 0.01. OD, optical density. |
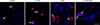 | Fig. 4The translocation of heat shock factor (HSF)-1 of chicken primary myocardial cells in response to geldanamycin (GA) and/or aspirin treatment. Chicken, primary myocardial cells, immunofluorescence staining, HSF-1 (red, TRITC), nuclei (blue, DAPI), HSF-1 and nuclei (pink, merged). ASA, acetylsalicylic acid. Scale bar = 20 µm. |
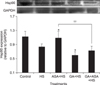 | Fig. 5Effect of different treatments on Hsp90 expression in chicken primary myocardial cells exposed to heat stress. The difference of the data of cells with different treatments (excluding the data of untreated cells) vs. that of heat-stressed cells are indicated by *p < 0.05. The difference of the data of cells treated by acetylsalicylic acid (ASA)+heat stress (HS) and geldanamycin (GA)+HS vs. that of cells treated by GA+ASA+HS is indicated by ††p < 0.01. |
 | Fig. 6Effect of different treatments on protein kinase B (Akt) and the signal transducer and activator of transcription (STAT)-3 expression in chicken primary myocardial cells exposed to heat stress. The difference of the data of cells with different treatments (excluding the data of untreated cells) vs. that of heat-stressed cells are indicated by **p < 0.01. The difference of the data of cells treated by acetylsalicylic acid (ASA)+heat stress (HS) and geldanamycin (GA)+HS vs. that of cells treated by GA+ ASA+HS is indicated by ††p < 0.01. |
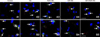 | Fig. 7The effect of different treatments on the co-localization of protein kinase B (Akt) and the signal transducer and activator of transcription (STAT)-3 with Hsp90 in chicken primary myocardial cells exposed to heat stress. Immunocytochemical staining. Hsp90 (red, TRITC), Akt/STAT-3 (green, FITC), nuclei (blue, DAPI), Hsp90 and Akt/STAT-3 (yellow, merged), Hsp90 plus Akt/STAT-3 and nuclei (near white, merged). Scale bars = 20 µm. |
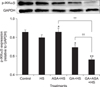 | Fig. 8The effect of different treatments on the p-IKKα/β level in chicken primary myocardial cells exposed to heat stress. The difference of the data of cells with different treatments (excluding the data of untreated cells) vs. that of heat-stressed cells are indicated by *p < 0.05 and by **p < 0.01. The difference between the data of cells treated by acetylsalicylic acid (ASA)+heat stress (HS) and geldanamycin (GA)+HS vs. that of cells treated by GA+ASA+HS is indicated by ††p < 0.01. |
 | Fig. 9The effect of different treatments on chicken primary myocardial cells exposed to heat stress. (A) The effect of heat stress (HS) on cell viability. (B) The effect of HS on cell apoptosis rate. (C) The effect of HS on cellular reactive oxygen species (ROS) level. The difference of the data of cells with different treatments (excluding the data of untreated cells) vs. that of heat-stressed cells are indicated by *p < 0.05 and by **p < 0.01. The difference of the data of cells treated by acetylsalicylic acid (ASA)+heat stress (HS) and geldanamycin (GA)+HS vs. that of cells treated by GA+ASA+HS is indicated by †p < 0.05 and by ††p < 0.01. |
 | Fig. 10The effect of different treatments on caspase-3, 8 and 9 activities in chicken primary myocardial cells exposed to heat stress. The difference of the data of cells with different treatments (excluding the data of untreated cells) vs. that of heat-stressed cells are indicated by *p < 0.05 and by **p < 0.01. The difference of the data of cells treated by acetylsalicylic acid (ASA)+heat stress (HS) and geldanamycin (GA)+HS vs. that of cells treated by GA+ASA+HS is indicated by †p < 0.05 and by ††p < 0.01. |
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31372403; 31672520), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Research Innovation Program of Graduate Students in Jiangsu Province (KYLX15-0536), the Fundamental Research Funds for the Central Universities (KJQN201709), the Natural Science Foundation of Jiangsu Province (BK20140107), and the Sino-German Agricultural Cooperation Project of the Federal Ministry of Food, Agriculture and Consumer Production, Berlin, Germany.
References
1. Amici C, Rossi A, Santoro GM. Aspirin enhances thermotolerance in human erythroleukemic cells: an effect associated with the modulation of the heat response of the heat shock responce. Cancer Res. 1995; 55:4452–4457.
2. Bagatell R, Beliakoff J, David CL, Marron MT, Whitesell L. Hsp90 inhibitors deplete key anti-apoptotic proteins in pediatric solid tumor cells and demonstrate synergistic anticancer activity with cisplatin. Int J Cancer. 2005; 113:179–188.

3. Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-κB activation. Oncogene. 2004; 23:5378–5386.

4. Chaudhury S, Welch TR, Blagg BSJ. Hsp90 as a target for drug development. ChemMedChem. 2006; 1:1331–1340.

5. Chávez-Mardones J, Gallardo-Escárate C. Immune response of apoptosis-related cysteine peptidases from the red abalone Haliotis rufescens (HrCas8 and HrCas3): molecular characterization and transcription expression. Fish Shellfish Immunol. 2014; 39:90–98.

6. Clark CB, Rane MJ, El Mehdi D, Miller CJ, Sachleben LR Jr, Gozal E. Role of oxidative stress in geldanamycin-induced cytotoxicity and disruption of Hsp90 signaling complex. Free Radic Biol Med. 2009; 47:1440–1449.

7. Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, Gonzalez-Gallego J. Quercetin decreases oxidative stress, NF-κB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005; 135:2299–2304.

8. Doong H, Rizzo K, Fang S, Kulpa V, Weissman AM, Kohn EC. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem. 2003; 278:28490–28500.

9. Faissner A, Heck N, Dobbertin A, Garwood J. DSD-1-proteoglycan/phosphacan and receptor protein tyrosine phosphatase-beta isoforms during development and regeneration of neural tissues. Adv Exp Med Biol. 2006; 557:25–53.

10. Feng J, Zhang M, Zheng S, Xie P, Ma A. Effects of high temperature on multiple parameters of broilers in vitro and in vivo. Poult Sci. 2008; 87:2133–2139.

11. Flandrin P, Guyotat D, Duval A, Cornillon J, Tavernier E, Nadal N, Campos L. Significance of heat-shock protein (HSP) 90 expression in acute myeloid leukemia cells. Cell Stress Chaperones. 2008; 13:357–364.

12. Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res. 2002; 90:866–873.

13. Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006; 25:4798–4811.

14. Giardina C, Lis JT. Sodium salicylate and yeast heat shock gene transcription. J Biol Chem. 1995; 270:10369–10372.

15. Gu XH, Hao Y, Wang XL. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers. 2. Intestinal oxidative stress. Poult Sci. 2012; 91:790–799.

16. Hao Y, Gu XH. Effects of heat shock protein 90 expression on pectoralis major oxidation in broilers exposed to acute heat stress. Poult Sci. 2014; 93:2709–2717.

17. Hatada EN, Krappmann D, Scheidereit C. NF-κB and the innate immune response. Curr Opin Immunol. 2000; 12:52–58.
18. Ikeyama S, Kokkonen G, Shack S, Wang XT, Holbrook NJ. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. FASEB J. 2002; 16:114–116.

19. Islam A, Lv YJ, Abdelnasir A, Rehana B, Liu ZJ, Zhang M, Tang S, Cheng YF, Chen HB, Hartung J, Bao ED. The role of Hsp90α in heat-induced apoptosis and cell damage in primary myocardial cell cultures of neonatal rats. Genet Mol Res. 2013; 12:6080–6091.

20. Jin M, Otaka M, Okuyama A, Itoh S, Otani S, Odashima M, Iwabuchi A, Konishi N, Wada I, Pacheco I, Itoh H, Tashima Y, Masamune O, Watanabe S. Association of 72-kDa heat shock protein expression with adaptation to aspirin in rat gastric mucosa. Dig Dis Sci. 1999; 44:1401–1407.
21. Jurivich DA, Pachetti C, Qiu L, Welk JF. Salicylate triggers heat shock factor differently than heat. J Biol Chem. 1995; 270:24489–24495.

22. Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C. Characterization of the signal that directs Bcl-xL, but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol. 2003; 160:53–64.

23. Koo HN, Oh SY, Kang KI, Moon DY, Kim HD, Kang HS. Modulation of HSP70 and HSP90 expression by sodium salicylate and aspirin in fish cell line CHSE-214. Zoolog Sci. 2000; 17:1275–1282.

24. Lee KH, Jang Y, Chung JH. Heat shock protein 90 regulates IκB kinase complex and NF-κB activation in angiotensin II-induced cardiac cell hypertrophy. Exp Mol Med. 2010; 42:703–711.

25. Liu CM, Zheng G, Ming QL, Chao C, Sun JM. Sesamin protects mouse liver against nickel-induced oxidative DNA damage and apoptosis by the PI3K-Akt pathway. J Agric Food Chem. 2013; 61:1146–1154.

26. Mohan S, Konopinski R, Yan B, Centonze VE, Natarajan M. High glucose-induced IKK-Hsp-90 interaction contributes to endothelial dysfunction. Am J Physiol Cell Physiol. 2009; 296:C182–C192.

27. Mujahid A, Pumford NR, Bottje W, Nakagawa K, Miyazawa T, Akiba Y, Toyomizu M. Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. Poult Sci. 2007; 44:439–445.

29. Padmini E, Usha Rani RM. Heat-shock protein 90 alpha
(HSP90α) modulates signaling pathways towards tolerance
of oxidative stress and enhanced survival of hepatocytes of
Mugil cephalus. Cell Stress Chaperones. 2011; 16:411–425.

30. Shimp SK 3rd, Parson CD, Regna NL, Thomas AN, Chafin CB, Reilly CM, Nichole Rylander M. HSP90 inhibition by 17-DMAG reduces inflammation in J774 macrophages through suppression of Akt and nuclear factor- κB pathways. Inflamm Res. 2012; 61:521–533.

31. Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000; 97:10832–10837.

32. Schoof N, von Bonin F, Trümper L, Kube D. HSP90 is essential for Jak-STAT signaling in classical Hodgkin lymphoma cells. Cell Commun Signal. 2009; 7:17.

33. Shang L, Tomasi TB. The heat shock protein 90-CDC37 chaperone complex is required for signaling by types I and II interferons. J Biol Chem. 2006; 281:1876–1884.

34. Shen HY, He JC, Wang Y, Huang QY, Chen JF. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem. 2005; 280:39962–39969.

35. Wu T, Mohan C. The AKT axis as a therapeutic target in autoimmune diseases. Endocr Metab Immune Disord Drug Targets. 2009; 9:145–150.

36. Zhang H, Chung D, Yang YC, Neely L, Tsurumoto S, Fan J, Zhang L, Biamonte M, Brekken J, Lundgren K, Burrows F. Identification of new biomarkers for clinical trials of Hsp90 inhibitors. Mol Cancer Ther. 2006; 5:1256–1264.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download