Abstract
Recently, we reported that Artemisia annua (AA) has anti-adipogenic properties in vitro and in vivo. Reduction of adipogenesis by AA treatment may dampen systemic inflammation and protect neurons from cytokine-induced damage. Therefore, the present study was undertaken to assess whether AA increases neuronal maturation by reducing inflammatory responses, such as those mediated by cyclooxygenase 2 (COX-2). Mice were fed normal chow or a high-fat diet with or without chronic daily oral administration of AA extract (0.2 g/10 mL/kg) for 4 weeks; then, changes in their hippocampal dentate gyri were measured via immunohistochemistry/immunofluorescence staining for bromodexoxyuridine, doublecortin, and neuronal nuclei, markers of neuronal maturation, and quantitative western blotting for COX-2 and Iba-1, in order to assess correlations between systemic inflammation (interleukin-6) and food type. Additionally, we tested the effect of AA in an Alzheimer's disease model of Caenorhabditis elegans and uncovered a potential benefit. The results show that chronic AA dosing significantly increases neuronal maturation, particularly in the high-fat diet group. This effect was seen in the absence of any changes in COX-2 levels in mice given the same type of food, pointing to the possibility of alternate anti-inflammatory pathways in the stimulation of neurogenesis and neuro-maturation in a background of obesity.
Artemisia annua (AA), a well-known anti-malarial agent [1120], has recently been reported to have anti-adipogenic effects in vitro [11] and in vivo [2]. Adipose tissue plays important roles in energy metabolism, thermoregulation, and the production of key adipokines and cytokines [671719]. However, consumption of high-fat or high-calorie foods can lead to obesity and diabetes, which both result from excessive adipogenesis. Many reports have demonstrated that chronic obesity and diabetic conditions lead to metabolic stress, possibly due to increased activity of macrophages and lymphocytes that contribute to a chronic state of low inflammation, characterized by the presence of proinflammatory factors such as interleukin-1 (IL-1) and interleukin-6 (IL-6) [1819]. This chronic inflammatory state can have a harmful effect on the hippocampus, learning, cognition, and memory, and may lead to dementia and depression [121].
We previously reported that AA can reduce adipogenesis and de-accelerate weight gain when chronically administered in a diet-induced obesity (DIO) mouse model. This finding supports in vitro data using 3T3-L1 cells [2]. From that study, we hypothesized that chronic oral administration of AA can inhibit adipogenic effects by increasing neuronal differentiation and maturation. Interestingly, Nam et al. [13] reported that inducible cyclooxygenase 2 (COX-2) was closely associated with neuroinflammation. COX-2 has also been associated with changes in hippocampal synaptic plasticity [422], along with effects on neuronal stem cell populations, cell proliferation, neuronal differentiation, memory formation, and Alzheimer's disease (AD) [813]. In this study, we asked whether AA treatment in a DIO mouse model can increase neuronal differentiation and maturation by regulating COX-2 expression in the hippocampal dentate gyrus.
Preparation of AA was performed according to a method published in our previous report [2]. Briefly, a total of 40 g of AA was heated for 30 min with 1.8 L of distilled water (DW) under 150,000 Pa at 80℃. The extract was allowed to fully cool, and then was filtered first with paper (185 mm; Advantec, Japan) and then with a Nalgene Rapid-Flow Bottle Top Filter (0.2 µm pore membrane; Thermo Scientific, USA). The final AA extract was stored at −80℃ until use.
Twenty-four adult C57BL/6J mice (mean weight, 23.0 g; 7-week-old) were maintained at room temperature (23 ± 1℃) and 60% humidity under a 12 h light-dark cycle (light cycle from 7:00–19:00). The mice were separated into four groups, two that were provided a normal chow diet (ND) (2018S; Harlan, USA) and two that were given a high-fat diet (HD) (2018S; Harlan). All animals had ad libitum access to water. The AA extract (0.2 g AA/10 mL DW/kg) was carefully administered with an oral sonde (0.9 × 50 mm) to half of each food group, while the same amount of DW was administered to the other half. Body weight and food intake were recorded daily.
Mice were treated with 5′-bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich, USA) at 7 weeks of age in order to label endogenous proliferating cells. BrdU (50 mg/kg) was administered intraperitoneally every 12 h for 3 days. The mice were sacrificed 28 days following the last BrdU injection, as it takes approximately 4 weeks for newly developed neurons to differentiate in adult mice.
All procedures involving animals were done in accordance with the ethical standards of the Institutional Animal Care and Use Committee (IACUC approval No. SCH15-0001) at Soonchunhyang University.
Blood samples were collected from the heart and centrifuged at 15,000 × g for 10 min. Then, the IL-6 level in serum was measured with a Mouse IL-6 ELISA Kit (Koma Biotech, Korea). We performed the test according to the instructions provided with the kit.
For Immunohistochemistry, brain tissue sections between −1.46 and −2.47 mm were made using the Bregma anatomical reference in mouse [16] for each animal. The sections were incubated overnight with primary antibodies specific for DCX (1:100; Santa Cruz Biotechnology, USA), and Iba-1 (1:1,000; Chemicon, USA). Sequentially, the sections were incubated with biotinylated secondary antibodies and streptavidin peroxidase complex (Vector Labs, USA). Antibody bindings were detected with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich). The number of positive DCX or Iba-1 cells for all groups were noted. In particular, the number of DCX-positive cell bodies as neuroblasts or DCX_Blasts were counted in the subgranular zone (SGZ) of the hippocampal dentate gyrus, and DCX-positive dendrites from the neuroblasts were also counted in the granular cell layer (GCL) and the molecular layer (ML) in the hippocampal dentate gyrus.
Brains were removed from animals and the hippocampal tissues were separated. All tissues were frozen with liquid nitrogen and homogenized in PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Korea), and protein concentrations were determined with a SMART BCA kit (iNtRON Biotechnology). Lysates were separated with 10% SDS-PAGE and then transferred to PVDF membranes (Bio-Rad Laboratories, USA). The membranes were probed with primary antibodies against COX-2 (Cayman, USA), Iba-1 (Wako, Japan), and GAPDH (Cell Signaling Technologies, USA), then incubated overnight. After further washing, membranes were incubated with HRP-conjugated secondary antibody (Vector Labs, USA) and ECL substrate solution (Progma, USA). CheBi (Neoscience, Korea) was used for imaging.
For immunohistochemical analysis, brain tissue sections between −1.46 and −2.47 mm were made using the Bregma anatomical reference [16] for each animal. The sections were incubated overnight with primary antibodies specific for DCX (1:500; Santa Cruz Biotechnology). Sequentially, the sections were incubated with biotinylated secondary antibodies and streptavidin-peroxidase complex (Vector Labs). Antibody binding was detected with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich). The number of DCX-positive cells in all groups were recorded. Of special importance, the number of DCX-positive cell bodies as neuroblasts or DCX-Blasts were counted in the SGZ of the hippocampal dentate gyrus, and DCX-positive dendrites from the neuroblasts were also counted in the GCL and ML in the hippocampal dentate gyrus.
Brain tissues were cryoprotected by infiltration with 30% sucrose in phosphate buffer (pH 7.2). The frozen tissues were then serially sectioned on a cryostat (Thermo Scientific, USA) in 30 µm coronal sections containing the dentate gyrus of the hippocampus according to anatomical landmarks given by Bregma (−1.46 mm to −2.46 mm) in the mouse brain atlas [17] and they were then transferred to 24-well plates containing phosphate buffered saline (PBS).
Double immunofluorescence staining of the tissue samples was performed to confirm the presence of differentiating neuronal cells stained with DCX (a neuroblast marker), as well as differentiation from newly-generated cells stained with BrdU at the beginning of AA administration into mature neurons with NeuN staining (a neuronal nucleus marker). Double staining for cells with both BrdU and DCX signals was also performed. For BrdU and antibody staining of the sections, we incubated for 2 h in 50% formamide/2× SCC (0.3M NaCl, 0.03M sodium citrate) at 65℃, followed by incubation for 30 min in 2N HCl at 37℃, and then by a 10 min rinse in 0.1 M boric acid (pH 8.5). Following this, the sections were incubated in a mixture of goat anti-DCX (diluted 1:200, Santa Cruz Biotechnology)/rabbit anti-NeuN (1:5,000, Millipore, USA), rat anti-BrdU (diluted 1:400, Santa Cruz Biotechnology)/rabbit anti-NeuN (1:5,000), and a mixture of rat anti-BrdU (diluted 1:400)/goat anti-DCX (diluted 1:200) overnight at 4℃. They were then incubated in a mixture of Cy3-conjugated AffiniPure donkey anti-rat IgG (1:300, Jackson ImmunoResearch, USA) for BrdU staining, Alexa Fluor 488 donkey anti-goat IgG (1:300, Invitrogen, USA), and Alexa Fluor 594 donkey anti-goat IgG for DCX staining, and Alexa Fluor 488 donkey anti-rabbit IgG (1:300, Invitrogen) for NeuN staining for 2 h at room temperature. Immunoreactivity was observed with a fluorescence microscope (Olympus, USA) after the slides were mounted and coverslipped with Vector Shield media (Vector Labs). The DCX/NeuN double-merged images, BrdU/NeuN double-merged images and BrdU/DCX double-merged images were captured with the image analysis program in Meta Imaging series 7.1 software (Molecular Devices, USA).
The C. elegans CL4176 strain expressing muscle-specific human Aβ1–42 [dvls27 (myo-3/Aβ1–42/let UTR, rol-6)] was purchased from CGC (USA). Worms were cultured at 20℃ on NGM (Nematode Growth Media) agar plates (1.7% agar, 2.5 mg/mL peptone, 25 mM NaCl, 50 mM KH2PO4 pH 6.0, 5 µg/mL cholesterol, 1 mM CaCl2, and 1 mM MgSO4) seeded with Escherichia coli OP50 as the food source. For the paralysis assay, five CL4176 L4/young adult worms were transferred to a fresh NGM plate and were allowed to lay eggs for 2 h at 15℃. After eliminating the five adult worms, eggs were maintained at 15℃ for 5 days. Age-synchronized young adult worms were allowed to lay eggs on NGM plates spread with 100 µL of AA extract for 2 h at 15℃. The gravid adults were removed from the plate and the progeny were upshifted to 25℃ to induce Aβ peptide production. The number of paralyzed worms was scored every hour for 24 h after the temperature upshift. The nematodes were scored every hour until they were all paralyzed. To identify paralysis, each nematode was touched gently with a platinum loop. The nematode was considered paralyzed if it moved its head slightly or did not move at all after being touched. The numbers of paralyzed worms were compared between untreated control and AA-treated groups. The log-rank test was used for statistical analysis asymptotically efficient rank invariant test procedures were used as previously reported [16].
To ensure objectivity, all measurements were performed under blinded conditions by two observers per experiment and under identical conditions. For quantitation of immunoreactivity, the extent of the staining was measured in five sections per animal. The images of DCX, BrdU, and NeuN immunoreactive structures were taken by using a BX53 light microscope (Olympus) equipped with a digital camera (DP71, Olympus) connected to a personal computer and a monitor.
The data shown represent the experimental mean ± SE for each experimental group. Differences between means were analyzed by using repeated two-way analysis of variance and one-way analysis of variance followed by Bonferroni post hoc test and Duncan's new multiple range tests in order to determine differences between experimental groups. The measures were analyzed statistically by ANOVAs (with repeated measure when appropriate). Statistical analyses were performed by using JMP9 (SAS Institute, USA). P values < 0.05 were considered statistically significant.
Body weight measurement results showed that the HD/AA group weighed significantly less than did the HD/DW group; in contrast, the ND/DW and the ND/AA groups did not show any significant weight differences. The weight difference between the HD/AA and HD/DW groups began on day 16 following AA treatment and was maintained as the experiment progressed (panel A in Fig. 1). There were no notable differences in the first two weeks for the HD-fed groups.
On the other hand, food intake in both ND-fed groups (ND/DW and ND/AA) and in both HD-fed groups (HD/DW and HD/AA) did not show any significant differences throughout the experimental period (panel B in Fig. 1). There was a lower IL-6 level in the AA-administered group than in the DW-treated group in the HD-fed mice (Fig. 2). Serum of HD-fed mice showed higher IL-6 levels than did that of ND-fed mice when administered DW. However, the IL-6 level in AA-administered mice was much lower than that in DW mice when fed HD. The IL-6 levels in the ND-fed groups were similar, regardless of substance administered (Fig. 2).
The Iba-1 level of AA-administered mice was considerably lower than that in DW-administered mice (panels A and B in Fig. 3). However, this tendency was not observed in the western blot results. The Iba-1 positive cells abundance was significantly lower in the AA administered-groups than in the DW-treated groups in both ND and HD food types (panel B in Fig. 3).
Quantitation of inflammatory factors relative to GAPDH was performed by using western blots (panel C in Fig. 3). COX-2 and Iba-1 expression in the hippocampus of HD-fed mice were higher than the levels in ND-fed mice (panels D and E in Fig. 3). Overall, the expression of Iba-1 was slightly higher than that of COX-2 (p<0.05).
DCX immunoreactivity represents neuronal differentiation and the presence of neuroblasts. DCX-positive signals by DAB chromogen were shown in the hippocampal dentate gyrus. Neuroblasts (“DCX_Blast”) (panel B in Fig. 4) and dendrites from the DCX-positive cell bodies (“DCX_Branch”) were analyzed (panel C in Fig. 4). As shown in Fig. 4, DCX_Blast and DCX_Branch levels were higher in the AA-treated groups than in the DW-treated groups for both food-type groups. There were no significant differences between ND/DW and ND/AA in the DCX_Blast and DCX_Branch groups in the hippocampal dentate gyrus. The number of secondary- and tertiary-branched dendrites showed significant differences only between the HD/DW and HD/AA groups (p<0.05).
To investigate the extent of neuronal differentiation, neurons were double-stained with NeuN and DCX and labeled with BrdU in the hippocampal dentate gyrus. As shown in panels A and B in Fig. 5, DCX-positive cells were observed in the SGZ and GCL in the dentate gyrus in the AA-treated groups on ND and HD diets (panel B in Fig. 5; arrows). However, the length of dendrites, as assessed by DCX immunofluorescence, in the HD/DW group was shorter than that in the ND/DW group. For these experiments, BrdU was injected systemically at the beginning of AA administration, and BrdU-labeled cells (in red) were seen in the SGZ and GCL areas, while cells positive for NeuN were observed in the AA-treated groups in both ND and HD groups, but particularly in the HD/AA group (panel D in Fig. 5; arrows). The BrdU-positive cells had migrated into the GCL from the SGZ. Panels G and H in Fig. 5, respectively, show the number of BrdU-positive neurons in the whole hippocampus and in the GCL. In the hippocampus, BrdU expression in HD-fed mice was lower than that of ND-mice; however, in the HD-fed group, it appeared that AA-administered mice had a higher number of BrdU-positive cells (panel G in Fig. 5). Interestingly, and particularly in the GCL region, we observed that BrdU expression of AA-treated mice was notably increased over that of DW-treated mice, regardless of food type (panel H in Fig. 5).
A reliable and convenient screening for potential anti-AD agents uses the C. elegans paralysis and Aβ induction model. Compared to the DW-treated group (control), the AA-treated C. elegans group (AA) had a significantly higher percentage of “not paralyzed” animals (Fig. 6). In addition, the time (days) to paralysis after Aβ induction was significantly longer in the AA-treated group than in the control group.
The regenerative capacity of neurons has been recognized as a key component of human and mammalian physiology, and such regeneration may be linked to parameters affecting health and aging. Age, obesity, and diabetes are risk factors that can affect brain function by mechanisms such as inhibition of neurogenesis in the hippocampus [28912]. Metabolic disorders are considered to promote brain dysfunction and lead to dementia, depression, AD, and brain ischemia. In addition, metabolic disorders can lead to adipogenesis and increased inflammatory responses [2323], as well as to systemic insulin and leptin resistance. Therefore, combating aging, obesity, and diabetes may have positive effects on enhancing neurotrophic environments and maintaining proper brain health and function.
Interestingly, the discovery and development of AA as an anti-malarial agent was recently recognized via the awarding of the 2015 Nobel Prize in Physiology or Medicine to the scientist responsible, and we have reported that AA has anti-adipogenic effects [2]. As such, AA can reduce the inflammatory response, as well as insulin and leptin resistance, and it can have a neurotrophic effect on the brain, according to various theories [25]. We hypothesized that chronic AA administration might increase neurogenesis in the hippocampus.
A previous report by our research group showed that AA had the ability to reduce lipid droplet size increases in a HD background in vivo, and treatment with AA allowed adipose cells to remain undifferentiated in vitro [210]. We expected that there might be alterations in the brain, such as neuronal differentiation changes in the hippocampus, related to the presence of infiltrated immune cells, including macrophages and lymphocytes, in the interstitial region of the lipid droplets.
The IL-6 levels were greater in our HD/DW group than in either the ND/DW or ND/AA groups. This result is similar to previous findings. In the HD-fed mice, however, AA extract significantly decreased the inflammatory factor levels. It seems that AA can moderate chronic inflammatory reactions by suppressing excess adipogenesis. Interestingly, this tendency was not observed in the hippocampus. Western blot results demonstrated that COX-2 and Iba-1 expressions were not decreased by AA extract. This discrepancy may be due to the following. The Iba-1 positive cells were quantitatively counted in the hippocampus between −1.46 and −2.47 mm of the Bregma anatomical reference for each animal. The scope of that area is only a portion of the whole hippocampus. Since the western blot results were based on Iba-1 expression of the whole hippocampus, the quantitatively measured hippocampal Iba-1-positive cell counts from only a portion of the hippocampus may have produced different results.
We suggest that moderation of the inflammatory reaction in the body might have a positive effect on brain function. However, the COX-2 pathway is not essential for the promotion of neuronal maturation following treatment with AA extract. Although multiple studies have reported that COX-2 has a pivotal role in enhancing neurogenesis in the hippocampus [131415], our western blot results showed that COX-2 expression in the hippocampus did not significantly change with chronic AA administration in mice fed either of the diets. It may be that AA does not affect the COX-2-related pathways, but that an alternative signaling pathway enhances neurogenesis. This speculation is strengthened by the observation that the number of Iba-1-positive cells was not reduced in mice fed either type of food and treated with AA.
Our results show that mice treated with AA had alterations in the levels of neurogenesis under both ND or HD diets. The number of neuroblasts did not significantly change with respect to AA treatment, but it did change with respect to food type. However, the number of neuronal branches (secondary and tertiary dendrites) stemming from the neuroblasts was slightly greater in the HD/AA group than in the HD/DW group; there were no significant differences between the ND/AA and ND/DW groups.
In this study, we focused on how many new neuronal cells matured since the beginning of the experiment, rather than on simply determining whether neurogenesis increased. Chronic administration of AA led to increased differentiation of cells to neuroblasts and neurons in mice fed both types of food, as shown in Fig. 5. DCX-positive cells had their dendrites double-stained with the NeuN neuronal marker in the GCL area in the hippocampus. The number of double-positive cells was significantly increased in the AA-treated groups given either food type. In addition, many BrdU-labeled cells migrated from the SGZ to the GCL in the AA-treated groups given either food type.
In the hippocampal tissue as a whole, BrdU expression in each group significantly differed by food type. As previous studies have reported, continuous intake of HD suppresses neurogenesis in the hippocampus. However, AA treatment is thought to promote differentiation of neurons by moderating the negative effects of a HD. This tendency was clear in the HD-fed group.
Also, interestingly, the effect of AA was obvious when focusing on the GCL region. We posit that an increase in BrdI-positive cells in the GCL region indicates that neuronal maturation occurs more actively there. This result is meaningful because the trend was evident in AA-administered mice, regardless of food type. Therefore, we suggest that AA is more related to the maturation of neurons than it is to their generation.
We also hypothesized that enhanced neurogenesis following AA treatment, even in the background of a HD diet, may provide an anti-AD benefit. We used the C. elegans paralysis model of AD to test this hypothesis. The AA-treated C. elegans had lower levels of paralysis than that in the DW-treated organisms. Based on that result, AA is a promising agent that may enhance brain function and have a role as an anti-AD agent.
In conclusion, although the systemic IL-6 level in the blood of the HD/AA group was significantly downregulated, interestingly, COX-2 and microglia (Iba-1) expressions were not different between DW- and AA-treated groups in the hippocampus, regardless of food type in the diet. However, neuronal maturation was significantly increased in the AA-treated groups given either food type, compared to the level of neurogenesis in the hippocampus.
In addition, the results verify that AA administration has an anti-AD effect in a C. elegans model, thus demonstrating the possibility of applying our results to future research into pathologic conditions. Given our initial findings in C. elegans, we intend to investigate the potential anti-AD benefit of AA in a higher animal model.
Figures and Tables
 | Fig. 1Changes in body weight (A) and food intake (B) in the four study groups (n = 5 per group). (A) Body weights in the normal chow diet (ND)/distilled water (DW) and ND/Artemisia annua (AA) groups were not significantly different during the treatment period; however, the body weights in the high-fat diet (HD)/DW and HD/AA groups were significant different at and after 16 days of oral AA administration. (*p<0.05; **p<0.01; ***p<0.005). (B) Interestingly, food intake did not significantly change with AA dosing in either the ND or HD group. |
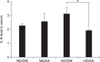 | Fig. 2Interluekin-6 levels in serum. In the normal chow diet (ND)-fed group, Artemisia annua (AA)-treated mice did not show any significant change in serum IL-6 level. The IL-6 level was significantly reduced in the high-fat diet (HD)/AA group compared with the level in the HD/distilled water group (*p<0.05). |
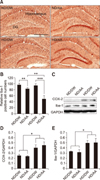 | Fig. 3Changes in inflammatory marker protein expressions of cyclooxygenase 2 (COX-2) and Iba-1 with respect to enhanced neuronal differentiation and neuronal maturation by chronic AA oral administration. The microglia populations stained using anti-Iba-1 antibody (A) were counted and the data were analyzed (B). The Artemisia annua (AA)-treated groups had decreased numbers of Iba-1 positive cells compared to those in the non-treated (distilled water [DW]) groups for each food group (normal chow diet [ND] and high-fat diet [HD]). The Iba-1 population changes did not show any significance between the ND/DW and HD/DW groups but showed a significant difference between the ND/AA and HD/AA groups (**p<0.01; ***p<0.005). The bands demonstrate COX-2 and Iba-1 expression according to group (C). The expressions of COX-2 (D) and Iba-1 (E) were significantly greater in the HD groups than in the ND groups. However, AA administration did not change the protein levels in either the ND or HD groups (*p<0.05). Scale bar = 200 µm. |
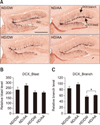 | Fig. 4Differences in neuronal differentiation in terms of neuroblast numbers following chronic AA oral administration. Doublecortin (DCX)-positive signals were observed in the hippocampal dentate gyrus (positive DAB color). Neuroblasts (“DCX_Blast”) were shown along the subgranular zone (SGZ) and their dendrites (“DCX_Branch”), which branched to the granular cell layer (GCL) and molecular layer (ML) (arrows) (A). The DCX_Blast numbers showed no significant difference between any of the four treatment groups (B). However, the DCX_Branch numbers were significantly different between the high-fat diet (HD)/distilled water (DW) and HD/Artemisia annua (AA) groups (C). Error bars indicate SE (*p<0.05). Scale bar = 200 µm. |
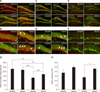 | Fig. 5Neuroblast numbers and differences in neuronal maturation resulting from chronic oral Artemisia annua (AA) administration. (A and B) Neuroblast marker doublecortin (DCX; red color) and the mature neuron marker neuronal nuclei (NeuN; green color) in the hippocampal dentate gyrus. (A) Samples were double stained with DCX/NeuN. (B) Magnified DCX/NeuN double-stained images (white boxes). As seen in panels A and B, the normal chow diet (ND) groups contained DCX-positive cells with more dendrites in the molecular layer (ML) than in the high-fat diet (HD) groups. The AA-treated groups (ND/AA and HD/AA) had more DCX-positive cells that migrated into the granular cell layer (GCL) than the amount in the non-treated groups (ND/distilled water [DW] and HD/DW). (C and D) Injected bromodeoxyuridine (BrdU) began integrating into the proliferating cells at the time of oral AA administration. The incorporation label is visualized by immunofluorescence (red color) in the hippocampal dentate gyrus. The red cells are mainly seen at the SGZ and the GCL in the dentate gyrus and are double-labeled with NeuN and BrdU. As seen in panel D, the magnified images (white boxes) demonstrate that the BrdU-positive cells became neurons and those cells arose from the proliferating cells at the beginning of AA administration (ND/AA and HD/AA groups); the double-labeled cells are colored yellow (D). The number of BrdU-positive cells was determined in the hippocampal dentate gyrus. Overall, the BrdU-positive cell counts were higher in the ND groups (ND/DW and ND/AA) than in the HD groups (HD/DW and HD/AA) (G). Meanwhile, BrdU-positive cell counts in the AA-treated groups fed both types of food were greater than those in the vehicle-treated groups (H). However, in the GCL region, the number of neurons stained with BrdU was significantly high in AA-treated mice in the HD group (H). Scale bars = 100 µm. |
 | Fig. 6Anti-Alzheimer's disease effects in the Caenorhabditis (C.) elegans paralysis (Aβ induction) model. The Artemisia annua (AA)-treated group exhibited less paralysis than that in the untreated group. Survival time in the AA-treated group was significantly longer than that in the untreated group. The control C. elegans group died within 7 days after the initiation of treatment; by comparison, the C. elegans AA-treated group survived an average of 14 days. |
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (project No. PJ01104602)” Rural Development Administration, Korea.
References
1. Aral LA, Pinar L, Göktaş G, Deveden EY, Erdoğan D. Comparison of hippocampal interleukin-6 immunoreactivity after exhaustive exercise in both exercise-trained and untrained rats. Turk J Med Sci. 2014; 44:560–568.

2. Baek HK, Shim H, Lim H, Shim M, Kim CK, Park SK, Lee YS, Song KD, Kim SJ, Yi SS. Anti-adipogenic effect of Artemisia annua in diet-induced-obesity mice model. J Vet Sci. 2015; 16:389–396.

3. Businaro R, Ippoliti F, Ricci S, Canitano N, Fuso A. Alzheimer's disease promotion by obesity: induced mechanisms-molecular links and perspectives. Curr Gerontol Geriatr Res. 2012; 2012:986823.

4. Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002; 87:2851–2857.

5. Doherty GH. Obesity and the ageing brain: could leptin play a role in neurodegeneration? Curr Gerontol Geriatr Res. 2011; 2011:708154.

6. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005; 115:911–919.

7. Frühbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol. 2008; 456:1–22.

8. Hoozemans JJ, Rozemuller AJ, Janssen I, De Groot CJ, Veerhuis R, Eikelenboom P. Cyclooxygenase expression in microglia and neurons in Alzheimer's disease and control brain. Acta Neuropathol. 2001; 101:2–8.

9. Hwang IK, Yi SS, Yoo KY, Park OK, Yan B, Song W, Won MH, Yoon YS, Seong JK. Differential effects of treadmill exercise in early and chronic diabetic stages on parvalbumin immunoreactivity in the hippocampus of a rat model of type 2 diabetes. Neurochem Res. 2011; 36:1526–1532.

10. Lee J, Kim MH, Lee JH, Jung E, Yoo ES, Park D. Artemisinic acid is a regulator of adipocyte differentiation and C/EBP δ expression. J Cell Biochem. 2012; 113:2488–2499.

11. Lee MR. Plants against malaria, part 2: Artemisia annua (qinghaosu or the sweet wormwood). J R Coll Physicians Edinb. 2002; 32:300–305.
12. Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006; 5:332–353.

13. Nam SM, Kim JW, Yoo DY, Choi JH, Kim W, Jung HY, Won MH, Hwang IK, Seong JK, Yoon YS. Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: effects on adult neurogenesis in the hippocampal dentate gyrus. J Vet Sci. 2015; 16:245–251.

14. Nam SM, Kim JW, Yoo DY, Choi JH, Kim W, Jung HY, Won MH, Hwang IK, Seong JK, Yoon YS. Effects of treadmill exercise on neural stem cells, cell proliferation, and neuroblast differentiation in the subgranular zone of the dentate gyrus in cyclooxygenase-2 knockout mice. Neurochem Res. 2013; 38:2559–2569.

15. Nam SM, Yi SS, Yoo DY, Kim W, Choi JH, Hwang IK, Seong JK, Yoon YS. Changes in cyclooxygenase-2 immunoreactivity in the hippocampus in a model of streptozotocin-induced type 1 diabetic rats. J Vet Med Sci. 2012; 74:977–982.

16. Park SK, Park SK. Electrolyzed-reduced water increases resistance to oxidative stress, fertility, and lifespan via insluin/IGF-1-like signal in C. elegans. Biol Res. 2013; 46:147–152.

17. Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 3rd ed. Elsevier Science;2004. Figs. 35-52.
18. Qian DX, Zhang HT, Ma X, Jiang XD, Xu RX. Comparison of the efficiencies of three neural induction protocols in human adipose stromal cells. Neurochem Res. 2010; 35:572–579.

19. Taupin V, Gogusev J, Descamps-Latscha B, Zavala F. Modulation of tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6, interleukin-8, and granulocyte/macrophage colony-stimulating factor expression in human monocytes by an endogenous anxiogenic benzodiazepine ligand, triakontatetraneuropeptide: evidence for a role of prostaglandins. Mol Pharmacol. 1993; 43:64–69.
20. Vereyken EJF, Bajova H, Chow S, de Graan PNE, Gruol DL. Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. Eur J Neurosci. 2007; 25:3605–3616.

21. Willcox M, Falquet J, Ferreira JF, Gilbert B, Hsu E, de Magalhães PM, Plaizier-Vercammen J, Sharma VP, Wright CW, Yaode W. RITAM Artemisia annua Task Force. Artemisia annua as a herbal tea for malaria. Afr J Tradit Complement Altern Med. 2006; 4:121–123.
22. Wu B, Hu K, Li S, Zhu J, Gu L, Shen H, Hambly BD, Bao S, Di W. Dihydroartiminisin inhibits the growth and metastasis of epithelial ovarian cancer. Oncol Rep. 2012; 27:101–108.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download