Abstract
A 14-year-old Maltese dog presented with progressive exophthalmos and external deviation of the right eye. Ultrasonography revealed the presence of a retrobulbar mass and fine-needle aspiration cytology was performed, which detected a malignant mass. There was no evidence of metastasis on thoracic and abdominal radiography. Computed tomography showed no invasion into the bony orbit and no metastasis to the lung or lymph nodes. Exenteration was performed to remove the mass completely. Malignant peripheral nerve sheath tumor was confirmed by histopathological examination.
Peripheral nerve sheath tumors (PNSTs) are a group of neoplasms arising from Schwann cells, perineural fibroblasts, or perineural cells [4511]. PNSTs are also known as schwannoma, neurilemmoma, neurogenic sarcoma, neurofibroma, or neurofibrosarcoma. These tumors are broadly classified as benign PNSTs or malignant PNSTs (MPNST) on the basis of cell morphology and biologic behavior [45]. Soft-tissue sarcomas, including MPNST, are typically reported as locally recurrent, rather than metastatic, and tend to occur in middleaged to older dogs [48]. MPNSTs involving the face are rare and cases associated with the eye have been scant in the veterinary literature [311]. Therefore, the purpose of this case report is to present a canine patient of MPNST that occurred within the orbit and in whom surgical excision was feasible and to describe the histopathological characteristics of canine MPNST. The prognosis for survival, surgical treatment, and outcome of orbital MPNST in dogs will also be discussed.
A 14-year-old castrated male Maltese dog was referred for a retrobulbar mass in the right eye (OD). The owner informed of progressive unilateral exophthalmos in the OD. A retrobulbar mass was considered suspicious on ultrasonography performed by the referring veterinarian. At first presentation, full ophthalmic examinations, including a tear production test (Schirmer Tear Test; Schering-Plough Animal Health, USA), neuro-ophthalmic examinations, applanation tonometry (Tono-Pen XL; Mentor, USA), slit lamp biomicroscopy (SP-2000P; Topcon, Japan) and indirect ophthalmoscopy, were performed. Menace response, direct and consensual pupillary light reflexes, and dazzle reflex were present in both eyes (OU). The eye globe was moderately exophthalmic OD, without apparent pain even while opening the mouth. There was mild lateral strabismus and mild conjunctival hyperemia and chemosis OD. In the fundic examination, mild vitreous and retinal degeneration OD were observed. No additional abnormalities were detected.
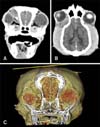
There were no significant abnormalities in the complete blood count and serum biochemical analysis. No evidence of metastasis was found on the thoracic and abdominal radiographic imaging. An irregular hypodense soft-tissue mass with surrounding contrast-enhancing tissue was detected on computed tomography (CT) images (Fig. 1). The mass was located dorsal, medial, ventral, and posterior to the right globe, potentially leading to the lateral strabismus and exophthalmos OD. There was no clear involvement of bones and lymph nodes adjacent to the soft-tissue mass and the lung parenchyma on the CT findings. Fine-needle aspiration (FNA) was performed following the CT to estimate the probability of malignancy or necrosis in the mass. High cellularity was shown in a cytologic evaluation of the FNA aspirates. The nucleated cells were almost all (> 99%) mesenchymal origin cells exhibiting malignant features.
The patient underwent surgical excision of the retrobulbar mass 5 days later. The dog was premedicated with cephradine (25 mg/kg intravenous [IV]; Cefradine Injection; Shin Poong Pharm, Korea), meloxicam (0.2 mg/kg subcutaneous; Metacam Solution for Injection; Boehringer Ingelheim Vetmedica, Germany) and acepromazine (0.1 mg/kg IV; SEDAJECT injection; Samu Median, Korea). General anesthesia was induced with propofol (6 mg/kg IV; Provive 1%; Myungmoon Pharm, Korea) and maintained with isoflurane (Ifran Solution; Hana Pharm, Korea). The surgical site was prepared aseptically after the dog was positioned in left lateral recumbency. Exenteration was performed to ensure complete excision of the mass, including the available margins of the normal tissue. The surrounding muscles, including the temporal muscle, were also partially resected to minimize the risk of recurrence.
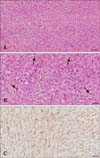
The surgically excised retrobulbar mass was immediately fixed in 10% neutral buffered formalin. The sample was routinely processed for histopathologic examination. The tissue sections were stained with hematoxylin and eosin. To clarify the origin of tumor cells, the streptavidin-biotin peroxidase complex immunohistochemistry (IHC) method was performed by using a neuron-specific enolase (NSE) antibody (monoclonal, M0873; Dako, Denmark). Histopathologically, the mass was unencapsulated and composed of neoplastic pleomorphic spindle cells. Tumor cells revealed an elongated shape or polyhedral pattern with high mitosis and were distributed in the fibrillar matrix (panel A in Fig. 2). Wavy spindle tumor cells were arranged in short bundles in both whorl and palisading form. Nuclei were central or eccentric with high mitotic figures (3–4 per high power field), and the cytoplasm was strongly eosinophilic and vacuolated (panel B in Fig. 2). Some tumor cells were loosely or densely arranged with crisscrossing or interlacing bundles and produced a large amount of mucinous material. Multifocal hemorrhage and necrosis were also observed throughout the mass. According to the IHC results, the tumor cells demonstrated strong positive reactions for NSE (panel C in Fig. 2). Based on the histopathologic characteristics, such as high mitosis, necrosis, hemorrhage, and the IHC results, this mass was diagnosed as MPNST.
Adjunctive chemotherapy was recommended, but refused by the owner. No apparent inflammation or pain around the surgical site was observed during a follow-up examination performed after one week. However, five weeks after surgery, the dog was presented to the referring veterinarian with swelling between the surgical site and the right ear. FNA was performed and recurrence of the tumor was identified. A second operation was considered for more extensive mass resection, but refused by the owner. The patient has been medicated with piroxicam (0.3 mg/kg per orally, once daily; Pillozen; Kolmar Pharma, Korea) by the local veterinarian as a potentially palliative option [6].
Orbital neoplasia was reported as the most common cause of orbital disease in canine and feline patients [2]. In dogs, primary ocular neoplasia is known to be more common than secondary ocular neoplasia, but uncommon compared with its occurrence in other organs [12]. It has been documented that orbital tumors in small animals have a tendency to be primary with malignancy [12] and occur in older females without breed predisposition, even though this study reports on the case of a castrated male dog [1]. Orbital tumors were reported to include 29 different tumor types, with tumors of mesenchymal origin being the most common [114]. The soft-tissue sarcomas are derived from non-bony connective tissues, differentiated by their histological appearance, and named after the connective tissue of origin [8]. These tumors have some similar biological and behavioral features of pseudo-encapsulation, with poorly defined margins, infiltration through fascial planes, common local recurrences after resection surgery, and unsatisfactory responses to radiation and chemotherapy [13]. The soft-tissue sarcomas generally exhibit a low metastatic rate of < 20%, while they are locally invasive. However, the histologically high-grade tumors were reported to show a tendency toward an increased metastatic rate of approximately 50% [813].
MPNST is a kind of soft-tissue sarcoma arising from the fibroblasts of the endoneurium, perineurium, epineurium, or the Schwann cells [34511]. In a human study, MPNST was reported as a rare tumor comprising 5% of all sarcomas, especially those involving the face, which are extremely rare [3]. The definitive diagnosis of this tumor was made through a biopsy and histopathological evaluation [138]. In veterinary medicine, PNST is also a rare tumor, reported to comprise just 0.5% to 2% of all skin tumors in dogs [9]. Intraocular PNSTs were documented by some recent case reports, with one report among them confirming the metastatic feature of this tumor [7]. Feline periocular PNST originated from the conjunctiva and eyelid [11] and canine orbital MPNST with bony invasion, in which surgical excision was not feasible, accompanied by same-sided temporal muscle atrophy and progressive neurological dysfunction for 1 month, resulting in the euthanasia of a 2-year-old dog [1], were also documented.
Carcinoma and sarcoma were reported to be the most common tumor types in canine and feline orbits, and both types have shown locally invasive behavior on magnetic resonance imaging results in a retrospective study [2]. This similarity of possible extensions into the surrounding tissues, observed as heterogeneous signal enhancement, has made it necessary to perform a biopsy and histopathologic examination to differentiate these tumors [28].
The histologic distinction between PNSTs and other spindle cell tumors, including fibrosarcoma, leiomyosarcoma, hemangiopericytoma, and melanoma, is known to be challenging, requiring immunohistochemical evaluation [4511]. The malignancy of PNSTs is determined on the basis of the presence of an increased mitotic rate, the degree of cellular anaplasia and pleomorphism, necrosis, and local invasion. Several histopathologic features, such as high mitosis, cellular pleomorphism, necrosis, and hemorrhagic foci, indicate that this retrobulbar mass is malignant. Based on the IHC, various markers, such as S-100 protein, glial fibrillary acidic protein, myelin basic protein, and NSE, have been extensively used to demonstrate cells originating from nerve sheath [511]. This method has also been applied to differentiate PNSTs from other spindle cell tumors. However, immunohistochemical studies have not made contributions to the establishment of clear diagnostic criteria for PNSTs [9]. In this case, MPNST showed a strong positive reaction to NSE and this result suggests that the retrobulbar mass is of neural origin. The IHC using the NSE marker has been widely used to identify neoplastic cells of the neuroectodermal origin [9].
Treatments for orbital tumors are determined by the presenting clinical signs, the types of tumors, the extent of their invasiveness, and the presence of metastatic lesions [11014]. In this case, complete physical examinations were performed, with very close attention devoted to enlargements of the lymph nodes following the observation of a suspicious retrobulbar mass through ultrasonography. Surgery could be planned because the thoracic and abdominal radiography and the CT revealed no evidence of invasion into the bony orbit or metastasis to the lung and regional lymph nodes. Exenteration of the entire contents of the orbit is the treatment of choice for orbital tumors, especially those affecting the posterior and ventromedial orbit, as in this case [10]. An attempt to resect the adjacent margins of normal tissue surrounding this tumor was undertaken during the exenteration. However, local recurrence occurred 5 weeks postoperatively in this dog, although an apparent complete gross excision of the tumor had been achieved. Soft-tissue sarcomas were reported to have a tendency to infiltrate fascial planes, opening the possibility of leaving microscopic amounts of tumor [8]. Chemotherapy might have been beneficial, but the owner declined further therapy. A feline retrospective study of periocular PNST also documented local aggressive recurrences similar to the tumor regrowth observed in this study [11]. After repeating surgery an average of three times, 2 of the 6 cats died, one underwent euthanasia 2 months postoperatively and the other died 3 months postoperatively, respectively, though exenteration had been performed.
Orbital MPNST is a highly malignant tumor with extremely invasive features and very low survival rates [14]. Initially performed wide and aggressive surgical excision in conjunction with exenteration should be the mainstay of orbital MPNST treatment [101314] because additional surgery following tumor recurrence could be needed to be more aggressive [811]. In orbital tumor, it is very hard to perform wide surgical excision involving enough margins in all dimensions, so that adjunctive chemotherapy, radiation therapy, or a combination of the two can be utilized following exenteration for orbital MPNST; such therapy might prolong life. A preoperative definitive diagnosis of MPNST, known as a tumor type showing common local aggressive recurrences, can help the surgeon to plan and choose more aggressive treatment methods, even considering the more radical orbitectomy required to prolong survival through complete tumor resection [81014]. Therefore, whenever possible, preoperative biopsy and histopathology to diagnose the tumor type are recommended.
Figures and Tables
Acknowledgments
This study was supported through BK21 PLUS Program for Creative Veterinary Science Research and the Research Institute for Veterinary Science (RIVS) of Seoul National University, Republic of Korea.
References
2. Armour MD, Broome M, Dell'Anna G, Blades NJ, Esson DW. A review of orbital and intracranial magnetic resonance imaging in 79 canine and 13 feline patients (2004-2010). Vet Ophthalmol. 2011; 14:215–226.

3. Aydin MD, Yildirim U, Gundogdu C, Dursun O, Uysal HH, Ozdikici M. Malignant peripheral nerve sheath tumor of the orbit: case report and literature review. Skull Base. 2004; 14:109–113.

4. Buza EL, Menzies RA, Goldschmidt MH, Durham AC. Malignant peripheral nerve sheath tumor in a cat with nodal and pulmonary metastases. J Vet Diagn Invest. 2012; 24:781–784.

5. Chijiwa K, Uchida K, Tateyama S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet Pathol. 2004; 41:307–318.

6. Ding H, Han C, Gibson-D'Ambrosio R, Steele VE, D'Ambrosio SM. Piroxicam selectively inhibits the growth of premalignant and malignant human oral cell lines by limiting their progression through the S phase and reducing the levels of cyclins and AP-1. Int J Cancer. 2003; 107:830–836.

7. Duke FD, Brudenall DK, Scott EM, Teixeira LBC, Dubielzig RR. Metastatic uveal schwannoma of blue-eyed dogs. Vet Ophthalmol. 2013; 16:Suppl 1. 141–144.

9. Gaitero L, Añor S, Fondevila D, Pumarola M. Canine cutaneous spindle cell tumours with features of peripheral nerve sheath tumours: a histopathological and immunohistochemical study. J Comp Pathol. 2008; 139:16–23.

10. Gelatt KN, Whitley RD. Surgery of the orbit. In : Gelatt KN, Gelatt JP, editors. Veterinary Ophthalmic Surgery. 2nd ed. St. Louis: Saunders Elsevier;2011. p. 51–88.
11. Hoffman A, Blocker T, Dubielzig R, Ehrhart EJ. Feline periocular peripheral nerve sheath tumor: a case series. Vet Ophthalmol. 2005; 8:153–158.

12. Labelle AL, Labelle P. Canine ocular neoplasia: a review. Vet Ophthalmol. 2013; 16:Suppl 1. 3–14.

13. MacEwan EG, Powers BE, Macy D. Soft tissue sarcomas. In : Withrow SJ, MacEwen EG, editors. Small Animal Clinical Oncology. 3rd ed. Philadelphia: WB Saunders;2001. p. 283–304.
14. Spiess BM, Pot SA. Diseases and surgery of the canine orbit. In : Gelatt KN, Gilger BC, Kern TJ, editors. Veterinary Ophthalmology. 5th ed. Ames: John Wiley & Sons;2013. p. 793–831.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download