A 7-month-old, 6.2 kg male intact West Highland white terrier was referred to the Wilford and Kate Bailey Small Animal Teaching Hospital at Auburn University for evaluation for balloon valvuloplasty for severe pulmonic stenosis. The dog had been diagnosed with a heart murmur on routine physical examination by his primary veterinarian, the etiology of which was determined to be severe pulmonic stenosis based on an echocardiogram performed by a non-specialist. Atenolol therapy was initiated at 1 mg/kg orally once daily. The dog was clinically normal with no history of weakness, collapse, or reduced exercise tolerance reported by the owner. Upon physical examination, a grade IV/VI left basilar systolic murmur was ausculted with a regular rhythm and a heart rate of 120 beats/min. No jugular venous pulsation was noted, and femoral pulse quality was normal.
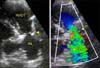
A two-dimensional echocardiographic image (Vivid E9; GE Healthcare, USA) was obtained from the right parasternal short axis view at the heart base. A discrete ridge of tissue was noted in the main pulmonary artery approximately 8 mm distal to the pulmonary valve, which was consistent with a supravalvular stenotic lesion (panel A in Fig. 1). There was marked post-stenotic dilatation of the main pulmonary artery. The internal diameter of the stenotic lesion was estimated to be 3 mm at its narrowest point, while the pulmonary valve annular diameter was 10 mm. Color flow Doppler examination demonstrated turbulent blood flow originating at the level of the supravalvular stenotic lesion (panel B in Fig. 1). The maximum continuous wave Doppler flow velocity through the stenotic area was 5.7 m/sec, which equated to a pressure gradient of 130 mmHg using the modified Bernoulli equation with aliasing of the pulsed wave Doppler profile at the supravalvular level. Supravalvular pulmonic stenosis was diagnosed based on two dimensional and Doppler echocardiographic studies. Severe right ventricular hypertrophy with flattening of the interventricular septum throughout the cardiac cycle and mild mitral regurgitation were also observed during echocardiographic examination. Based on these findings, balloon valvuloplasty was offered for therapeutic intervention.
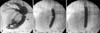
A 7 French percutaneous vascular sheath (Arrow International, USA) was placed at the right jugular via a modified Seldinger technique. A 6 French Berman angiographic catheter (Arrow International) was advanced through the vascular sheath into the right ventricle, after which 10 mL of Isovue-370 (Bracco Diagnostics, USA) was delivered by hand injection. Right ventricular angiography confirmed the location of the lesion and the degree of stenosis prior to balloon valvuloplasty (panel A in Fig. 2). A filling defect consistent with normal pulmonary valve leaflets was observed proximal to a discrete narrowing of the main pulmonary artery measuring 2.5 to 3 mm in diameter at its narrowest point. The pulmonary annular diameter was confirmed to be 10 mm. The features of the stenotic lesion were similar to those historically described in humans as “hour-glass deformity of the pulmonary valve,” in which the sinuses are bottle-shaped with narrow orifices and there is narrowing of the main pulmonary artery distal to the valve [12]. A 5 French pulmonary wedge pressure catheter (Arrow International) was used to measure the intra-operative pressures in the main pulmonary artery distal to the stenosis and in the right ventricle, with a systolic pressure gradient of 50 mmHg. A low profile balloon catheter (10 mm × 6 cm, 3 atmosphere burst pressure; Arrow International) was advanced across the stenotic lesion and rapidly inflated with diluted contrast solution by hand inflation. A discrete waist was noted at the level of the supravalvular stenosis (panel B in Fig. 2). Loss of the waist occurred with continued inflation (panel C in Fig. 2). Subsequent second inflation failed to show a discrete waist, suggesting successful alleviation of the stenosis. The intra-operative pressure gradient after successful balloon dilation revealed a systolic pressure gradient of 20 mmHg, showing a reduction of 60%.
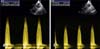
Transesophageal echocardiography (TEE; Multiplane Transesophageal Probe and Vivid E9 Ultrasound System; GE Healthcare) was performed before and after balloon dilation while the dog was under general anesthesia. The maximum continuous wave Doppler flow velocity through the stenotic area was 4.77 m/sec, which equated to a pressure gradient of approximately 90 mmHg prior to balloon dilation (panel A in Fig. 3). Immediately following balloon inflation, the maximal continuous wave Doppler flow velocity on TEE was 3.41 m/sec, which was equal to a pressure gradient of approximately 46 mmHg and a reduction in the pressure gradient of 49% (panel B in Fig. 3). Atenolol therapy was continued at 1 mg/kg orally twice daily for 2 months, then discontinued after tapering the dose over the course of 2 weeks. Follow-up echocardiography nine months after the procedure revealed a systolic pressure gradient of 60 mmHg across the lesion, showing a reduction of 54% when compared to the baseline. The degree of right ventricular concentric hypertrophy and post-stenotic dilation of the main pulmonary artery appeared to be static.
Pulmonic stenosis, which is narrowing from the right ventricular outflow tract to the main pulmonary artery, can be classified as valvular, subvalvular, or supravalvular [8]. Although congenital supravalvular pulmonic stenosis has been reported in dogs, it is rare [110]. Indeed, the only reported acquired supravalvular pulmonic stenosis in dogs was after subtotal pericardectomy and decortication to treat constrictive pericarditis [7].
Surgical correction of pulmonic stenosis, including open and closed valvotomy, closed and open patch graft techniques, and placement of a conduit, have all been described in veterinary medicine with mixed short term results [6]. Invasive surgical techniques have largely been supplanted by percutaneous balloon valvuloplasty, which is now considered the standard of care for pulmonic stenosis [3]. Balloon valvuloplasty is commonly performed for valvular pulmonic stenosis in veterinary medicine and is associated with good outcomes, especially in cases without pulmonary annular hypoplasia [8]. However, there is currently no standard therapeutic approach to supravalvular pulmonic stenosis in veterinary medicine.
In humans, balloon dilation of congenital supravalvular pulmonic stenosis may be unsuccessful because of elasticity and recoil of the sinotubular constriction, which characterizes this type of stenosis [24]. Balloon valvuloplasty has been attempted in cases of acquired supravalvular pulmonic stenosis, but has been similarly ineffective in some cases [14]. One case of supravalvular pulmonic stenosis occurring after an arterial switch operation underwent balloon valvuloplasty with a successful reduction in gradient, but this was followed by stent implantation because of continued evidence of hemodynamic compromise [9]. Endovascular stenting in cases of pulmonary artery stenosis is sometimes considered more effective than balloon dilation alone and is indicated for more diffuse lesions or those that do not respond adequately to balloon angioplasty [13].
There are few documented treatment options for supravalvular pulmonic stenosis in dogs. Although surgical techniques have been described with a few cases of good short-term success, these are invasive, technically challenging, and may be associated with higher morbidity and mortality than percutaneous procedures [15]. Unsuccessful reports of surgery for supravalvular pulmonic stenosis include an attempted arteriotomy and restenosis after patch graft placement [17]. Some surgical techniques also require cardiopulmonary bypass, which is not widely available in veterinary medicine. Transcatheter intravascular stent placement has been reported in two cases, both of which showed significant reduction in the pressure gradient across the stenotic area and resolution of clinical signs with a follow-up of 19 months and 2 years, respectively [7]. Moreover, successful balloon angioplasty for right pulmonary arterial branch stenosis was reported in a cat with a substantial reduction in the pressure gradient and clinical improvement [11]. To the best of our knowledge, balloon dilation alone has not previously been reported in dogs with supravalvular pulmonic stenosis.
Balloon valvuloplasty for valvular pulmonic stenosis, performed with a balloon diameter to annular ratio of 1.2 to 1.5, is considered effective and safe [5]. In humans, balloon dilation is performed for supravalvular pulmonic stenosis with balloon sizes ranging from 2 to 3 times the diameter of the narrowest segment, and not exceeding 1.3 times the pulmonary annulus diameter [14]. When a stent or balloon is used for peripheral pulmonary artery stenosis, it is selected to approximate the diameter of the vessel proximal and distal to the lesion [7]. We elected to use a balloon that approximated the size of the pulmonary annulus (10 mm), which was 3.5 to 4 times the diameter of the stenotic lesion. The balloon was inflated once with loss of waist noted, after which the post-procedure pressure gradient was reduced by 49% on transesophageal echocardiogram and at least 35% on transthoracic echocardiogram on immediate follow-up. Further studies are necessary to establish safe and effective balloon diameter to stenosis ratios; however, no complications were noted with a balloon diameter of over three times the stenotic lesion in this case. The use of balloons that approximate the size of the native vessel and are over twice the diameter of the stenotic lesion is well-established in human medicine [13]. The results of the present study show that balloon angioplasty, a widely available modality, is an appropriate treatment option for congenital supravalvular pulmonic stenosis in dogs. Failure of balloon dilation or restenosis may necessitate consideration of stent placement or surgical options.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download