Introduction
Chronic cerebral hypoperfusion (CCH) results in a marked and chronic reduction in cerebral blood flow, and CCH is closely associated with cognitive dysfunction and the onset of vascular dementia [620]. To investigate CCH and CCH-induced vascular dementia, a rat model with permanent bilateral common carotid arteries occlusion (2VO) was introduced and has been widely used [69192429]. The 2VO-induced CCH can lead to neuropathological alterations in some brain regions [562224]. Many previous studies have focused on CCH-induced neuropathological and neurochemical alterations in the hippocampus because the hippocampal CA1 region is one of the most vulnerable regions following CCH [168181924]. Although underlying mechanisms, related to CCH-induced neuronal damage, including oxidative stress and neuroinflammatory reactions [1818], have been suggested, the exact mechanisms explaining CCH-induced neuronal damage in the hippocampal CA1 region have not been fully elucidated.
Mammalian target of rapamycin (mTOR), a serine/threonine protein kinase, exists in two structurally and functionally distinct complexes: mTORC1 and mTORC2 [23]. These mTOR complexes are associated with various biological processes in cells, including regulation of protein synthesis, cellular proliferation and differentiation, and cell survival [41317]. In addition, it has been suggested that mTOR functions as a checkpoint to affect cell fate determination during autophagy, because enhanced activity of mTOR is thought to be a negative regulator of autophagy [1027]. Furthermore, it has been reported that cerebral ischemia causes a decrease in phosphorylated-mTOR (p-mTOR) and that the mTOR pathway has important and protective roles in controlling brain injury and neuronal death after ischemic insult [111221].
Although some investigators have described changes in the mTOR signal pathway in rat brain following CCH [92429], the roles and expressions of mTOR and p-mTOR in the brain following CCH remain unclear. In the present study, therefore, we examined temporal changes in mTOR and p-mTOR expressions in the rat hippocampal CA1 region following 2VO-induced CCH.
Materials and Methods
Experimental animals
Sixteen-weeks-old male Sprague-Dawley rats, obtained from RaonBio (Korea), were used for this experiment. All experimental procedures for animal handling and use were approved by Institutional Animal Care and Use Committee at Dankook University.
Surgery for chronic cerebral hypoperfusion
The surgical procedure for CCH was performed by executing 2VO surgery based on methods previously reported [818]. In brief, the animals were anesthetized with a mixture of 2.5% isoflurane (Baxter, USA) in 33% oxygen and 67% nitrous oxide. Both common carotid arteries were isolated through a midline cervical incision, and 2VO was performed with 5/0 silk suture. Rectal temperature was monitored and maintained by using a thermometric blanket before, during, and after 2VO surgery. Rats of the sham-operated group (sham-group) were subjected to the same surgical procedures but without performing 2VO. Rats that did not survive after 2VO surgery, were replaced.
Tissue processing
For histological analysis, tissue sections obtained from the sham- and CCH-operated animals (n = 6 each at 7, 14, 21, and 28 days after surgery) were examined. To obtain tissues, rats were anesthetized with zoletil 50 (30 mg/kg; Virbac, France) and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate-buffered saline. The brain tissues were removed, cryoprotected, and serially sectioned on a cryostat (Leica, Germany) into 30 µm coronal sections.
Cresyl violet staining for neuronal damage
To examine neuronal damage in the rat hippocampal CA1 region following CCH, cresyl violet (CV) staining was performed according to a method previously reported [16]. In brief, sections were stained with 1.0% (w/v) CV acetate (Sigma, USA), dehydrated, and then mounted with Canada balsam (Kanto, Japan).
To evaluate neuronal damage following CCH, digital images of the hippocampal CA1 region were captured by using an Axio Imager 2 microscope (Carl Zeiss, Germany) equipped with a digital camera (Axiocam; Carl Zeiss). According to the method described in our previous studies [1415], CV-positive (CV+) cells in the hippocampal CA1 region were counted in a 250 × 250 µm square at the center of the CA1 region. Six coronal sections at 120 µm intervals were selected per animal, and the number of CV+ cells was determined by averaging the counts from each animal.
Immunohistochemistry for mTOR and p-mTOR
According to the method described in our previous studies [14151619], immunohistochemistry for mTOR and p-mTOR was performed with rabbit anti-mTOR (1:100; Santa Cruz Biotechnology, USA) or rabbit anti-p-mTOR (ser2448) (1:100; Santa Cruz Biotechnology) as the primary antibody.
Eight sections at 120 µm intervals were selected per animal to quantitatively analyze mTOR and p-mTOR. Digital images of the hippocampal CA1 region were captured with an Axio Imager 2 microscope (Carl Zeiss) equipped with an Axiocam digital camera (Carl Zeiss) connected to a PC monitor.
Western blot analysis for mTOR and p-mTOR
To examine changes in mTOR and p-mTOR protein levels in the hippocampal CA1 region following CCH, the sham- and CCH-operated animals (n = 5 each at 7, 14, 21, and 28 days after surgery) were used for western blot analysis performed according to the method described in our previous studies [141519]. In brief, after sacrificing the animal and removing the brain, the brain was serially and transversely cut into 400 µm thick sections on a vibratome (VP1000P; Leica), and the hippocampal CA1 regions were then dissected with a surgical blade. The tissues were homogenized and centrifuged, and the obtained supernatants were subjected to western blot analysis. Rabbit anti-mTOR (1:200; Santa Cruz Biotechnology), rabbit anti-p-mTOR (1:200; Santa Cruz Biotechnology), or mouse anti-β-actin (1:5,000; Sigma) was used as the primary antibody. Western blot analysis was performed with three repetitions. After scanning the western blotting results, densitometric analysis of western blot bands was performed by using Image J 1.46 software (National Institutes of Health, USA), to measure relative optical density (ROD). Ratios of the ROD of CCH-operated groups were represented as percentage values, with the sham-group results designated as 100%.
Statistical analysis
The data are presented as mean ± SEM values. Differences among means were statistically analyzed by performing a two-way analysis of variance followed by post hoc Dunnett's tests to elucidate differences among the groups. Statistical significance was considered present at p < 0.05.
Results
Neuronal damage in the hippocampal CA1 region following CCH
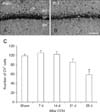
The CCH-induced neuronal damage in the hippocampal CA1 region was examined by CV staining. The CV+ cells in the stratum pyramidale (SP) of the hippocampal CA1 region were abundant in the sham-group (panels A and C in Fig. 1). Compared to the numbers in the sham-group, there were no significant differences in the numbers of CV+ cells in the SP at 7, 14, and 21 days following CCH in the CCH-group (panel C in Fig. 1). However, at 28 days following CCH, the number of CV+ cells were markedly and significantly decreased in the SP of the hippocampal CA1 region of the CCH-group (panels B and C in Fig. 1).
Changes in mTOR and p-mTOR immunoreactivity
In the sham-group, mTOR immunoreactivity was observed in the pyramidal neurons in the SP of the hippocampal CA1 region (panel A in Fig. 2). In the CCH-group, mTOR immunoreactivity in the SP was similar to that in the sham group at 7 and 14 days after 2VO surgery (panels B and C in Fig. 2). However, in the CCH-group, mTOR immunoreactivity began to decrease at 21 days following CCH (panels D and E in Fig. 2). At 28 days following CCH, a further decrease in mTOR immunoreactivity was detected in the SP of the hippocampal CA1 region of the CCH-group (panel E in Fig. 2).
The SP of the hippocampal CA1 region in the sham-group also exhibited p-mTOR immunoreactivity (panel A in Fig. 3). In the CCH group, p-mTOR immunoreactivity was markedly increased in the SP at 7 days following CCH (panel B in Fig. 3). Thereafter, p-mTOR immunoreactivity in the SP of the CCH-group decreased with time (panels C-E in Fig. 3). At 28 days following CCH, only a weak p-mTOR immunoreactivity was detected in the SP of the hippocampal CA1 region of the CCH-group (panel E in Fig. 3).
Changes in mTOR and p-mTOR protein levels
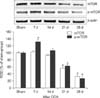
In the western blot analysis results, we observed that the patterns of changes in mTOR and p-mTOR protein levels in the hippocampal CA1 region after 2VO surgery were similar to those observed in the immunohistochemical results. The mTOR protein levels in the hippocampal CA1 region were significantly decreased at 21 and 28 days following CCH, compared to that in the sham group. In contrast, in the CCH-group, the p-mTOR protein level was significantly and transiently increased at 7 days after 2VO surgery; it then decreased with time after CCH (Fig. 4).
Discussion
In the present study, we detected a marked and significant decrease of CV+ pyramidal neurons in the SP of the hippocampal CA1 region cells at 28 days following CCH. Our results are consistent with those in previous studies, which reported that pyramidal neurons in the hippocampal CA1 region were indistinct and loosely arranged and that the number of pyramidal neurons was markedly reduced at 28 days after 2VO surgery [1924]. In addition, other previous studies have shown that marked neuronal damage in the hippocampal CA1 region occurs following CCH [16818].
A previous study showed that the number of primary cortical neurons was significantly increased in rapamycin-treated cultures over that in vehicle-treated cultures, following oxygen-glucose deprivation [7]. However, another study showed that inhibition of mTOR activity by rapamycin could reduce cell survival and increase apoptotic injury of hippocampal neurons during oxygen-glucose deprivation [3]. In addition, previous studies have shown that some pharmacological agents can reduce brain injury following focal cerebral ischemia via upregulation or maintenance of mTOR phosphorylation [111221]. It has also been suggested that improvement of mTOR activation may protect cerebral ischemic injury and enhance functional recovery [4]. Therefore, it has been generally accepted that mTOR has effective neuroprotective functions, although there is controversy regarding the effects of mTOR on ischemic injury [22526].
In this study, we observed that mTOR and p-mTOR protein expressions in the rat hippocampal CA1 region were markedly lower in the CCH-group than those in the sham-group, especially at 21 and 28 days after 2VO surgery. We speculated that there might be two possible mechanisms involved in the delayed reductions of mTOR and p-mTOR expressions. Firstly, decreased expressions of mTOR and p-mTOR were associated with neuronal death at 28 days after 2VO surgery. Secondly, significant reduction of mTOR and p-mTOR expressions at 21 days following CCH might be related to neuronal dysfunction, because we did not observe marked neuronal death at that time. In addition, our results support those in previous studies. One previous study reported that both the percentage of mTOR-immunopositive cells and the protein level of mTOR in the hippocampal CA1 region of rats with vascular dementia were significantly lower than those in the control group [24]. Another study showed that mTOR expression in the hippocampus was lower at two months after 2VO surgery than that in shamoperated rats [29]. The authors of that study suggested that reductive expression of mTOR in hippocampus might play an important role in the pathogenesis of vascular dementia [29]. In addition, a recent study showed that both mTOR and p-mTOR (ser2448) were downregulated in rat hippocampus at 20 days following CCH, although there was no significance to the variation in mTOR expression [9]. However, the authors suggested that CCH can affect gene expression and phosphorylation of mTOR [9]. Therefore, taken together with results in previous studies, our results show clearly that CCH leads to marked reductions of mTOR and p-mTOR expressions in the hippocampal CA1 region. Moreover, it is likely that the marked reductions of mTOR and p-mTOR expressions may be closely associated with CCH-induced neuronal damage/dysfunction in the hippocampal CA1 region and with the pathogenesis of vascular dementia.
Furthermore, in the present study, we found that p-mTOR protein expression was transiently and significantly increased in the hippocampal CA1 region at 7 days following CCH. To the best of our knowledge, this is the first report of such a result, and.it is hard to explain why p-mTOR expression would be transiently and significantly increased in the hippocampal CA1 region following CCH. A previous study showed no significant difference in total mTOR expression in the mouse hippocampus between sham- and transient global cerebral ischemia-operated groups, but p-mTOR expression was significantly increased at 1 day after transient global cerebral ischemia [28]. In addition, mTOR has been identified as a central regulator of cell survival in neurodegenerative diseases and ischemic stroke [41317]. Therefore, based on the results and arguments of previous studies, it can be postulated that the transient increase of p-mTOR expression observed in the present study may be related to its function in cell survival following CCH.
In summary, mTOR and p-mTOR protein expressions were reduced in the hippocampal CA1 region following CCH, although p-mTOR expression was transiently increased in the hippocampal CA1 region following CCH The results indicate that changes in mTOR and p-mTOR expressions in the hippocampal CA1 region might be closely related to CCH-induced neuronal damage in hippocampal CA1 pyramidal neurons.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download