Abstract
Excessive production of reactive oxygen species (ROS) and endoplasmic reticulum (ER) stress-mediated responses are critical to embryonic development in the challenging in vitro environment. ROS production increases during early embryonic development with the increase in protein requirements for cell survival and growth. The ER is a multifunctional cellular organelle responsible for protein folding, modification, and cellular homeostasis. ER stress is activated by a variety of factors including ROS. Such stress leads to activation of the adaptive unfolded protein response (UPR), which restores homeostasis. However, chronic stress can exceed the toleration level of the ER, resulting in cellular apoptosis. In this review, we briefly describe the generation and impact of ROS in preimplantation embryo development, the ROS-mediated activation mechanism of the UPR via the ER, and the subsequent activation of signaling pathways following ER stress in preimplantation embryos.
Development of economically important animals in laboratory environments can be critical for their successful survival, and in vitro-produced (IVP) embryos may determine the fate of large multi-cellular animals [25]. Poor in vitro development and uncontrolled growth are major obstacles to high-quality, large-scale production of IVP embryos [4060]. The main obstacles in IVP embryo development are the production of excessive free radicals and the exposure of embryos to oxidative stress [4277]. A preimplantation embryo is vulnerable to reactive oxygen species (ROS) [35]. In preimplantation embryos and during oocyte development, various enzymes and metabolic pathways produce endogenous ROS [225680]. Such ROS negatively affect the production, quality, and development of embryos; thus, it is important that the ROS level is low in embryo culture media [11]. ROS accumulation negatively affects protein synthesis and endoplasmic reticulum (ER) homeostasis in embryos [68]. When ROS production exceeds the antioxidant capacity of preimplantation embryos, oxidative stress occurs [2757], and oxidative stress suppresses the embryo's defense capacity against ROS [3]. Normally, the production of ROS is counterbalanced by antioxidants, such as glutathione, vitamins C and E, and enzymes (e.g., catalase, superoxide dismutase, and glutathione peroxides), which convert the lethal ROS into less damaging molecules [66]. Excessive production of ROS causes different types of cell injuries including disturbed metabolism, amino and nucleic acid oxidation, mitochondrial and ER dysfunction, adenosine triphosphate (ATP) depletion, and apoptosis [4977]. Bain and colleagues reported that developmental arrest and damage in embryos are associated with excessive production of ROS during in vitro development [5].
The ER is the major intracellular compartment responsible for protein folding and processing [76]. In eukaryotic cells, the ER is the first organelle in the secretory pathway. The ER is responsible for the production and modification of proteins and the accurate delivery of these proteins to target sites via the secretory pathway. All secretory proteins in the secretory pathway first enter the ER, where proper protein folding occurs [3440]. The term ER quality control (ERQC) refers to proper folding of proteins, which are then exported to the Golgi complex, while improperly folded proteins are retained in the ER to either complete correct folding or to be targeted for degradation via ER-associated degradation (ERAD) [18]. Accumulation of misfolded proteins in the ER disturbs ER functions, which ultimately triggers ER stress [53]. The response to ER stress is referred to as an unfolded protein response (UPR), which maintains cellular homeostasis; however, if the stress exceeds the tolerable level of the ER, apoptotic signaling is initiated [97].
A preimplantation embryo is vulnerable to a variety of cellular stresses in vitro [41]. Several obstacles need to be overcome to achieve successful in vitro embryo production. The most crucial issue is to make the in vitro environment similar to that of the oviduct and uterus [91]. In vivo, oocytes and embryo are able to resist oxidative stress as a result of the actions of antioxidants present in follicular fluid [64] or those produced by the embryo and the oviduct [20]. Because protein folding is an important process in cell survival, all cells have sophisticated mechanisms to ensure that proper protein folding occurs and to prevent the accumulation/aggregation of misfolded proteins [16]. Yoon et al. [85] reported that when the oxygen concentration is similar to the in vivo concentration, the chances of ER stress and UPR are minimal. Protein folding reactions depend on appropriate environmental, metabolic, and genetic conditions. Any stresses that interrupt protein folding are a threat to cell viability [132950]. In eukaryotic cells, all proteins that transit to the secretory pathway first enter the ER, where they are assembled and folded into multi-subunit complexes before transiting to the Golgi compartment [37]. A preimplantation embryo needs to replace maternal RNA with embryonic RNA, which requires extensive new protein synthesis, to continue its development [93]. These new proteins must be properly folded in the lumen of the ER in order to maintain preimplantation development. Various factors/processes that lead to an imbalance in the protein folding process in the lumen of the ER will activate the UPR, ultimately inhibiting blastocyst formation during preimplantation embryo development in vitro [6]. In this review, we will briefly summarize the role of the ER in the development of preimplantation embryos and the molecular pathways activated after ROS production.
ROS is a broad term that not only refers to oxygen radicals (superoxide and hydroxyl), but also to some non-radical derivatives of molecular oxygen (O2), such as hydrogen peroxide (H2O2) [26]. In embryos, the metabolism of molecular oxygen is important [3379], and the average consumption rate in morula- and blastocyst-stage bovine preimplantation embryos is 2 nL per embryo per hour [63]. In embryonic metabolic reactions, during the intermediate steps of oxygen reduction, ROS are formed from the superoxide anion radical O2−H2O2, and the hydroxyl radical OH− [24]. In blastocyst-stage rabbit preimplantation embryos, H2O2 and O2− are produced at the fourth and fifth days post-coitum and remain for the rest of the preimplantation period [51]. The production of H2O2 during mouse embryo development is higher in vitro than in vivo [22]. In in vitro culture, the level of ROS production is particularly important; an increased concentration of ROS results in oxidative stress and impaired mitochondrial function in germinal vesicle- and metaphase II-stage mouse oocytes [62].
The amount of ROS production differs among the various stages of embryo development. In mouse embryos, a large quantity of ROS is produced at two times: at fertilization and at the G2/M phase of the second cell cycle [58]. Oxidative stress contributes to the etiology of defects in embryonic development, and the production of ROS is triggered by embryonic metabolism and/or by the embryo's surroundings. Several enzymatic mechanisms are involved in the generation of ROS. The source of ROS and the irrelative contribution to the development of oxidative stress appear to differ depending on the species, the developmental stage, and the culturing conditions. There are several exogenous and endogenous factors and culture conditions that can enhance the production of ROS. Endogenous ROS is produced by various metabolic pathways and enzymes such as oxidative phosphorylation, β-nicotinamide adenine dinucleotide phosphate (β-NADPH) reduced oxidase, and xanthine oxidase (XO). Exogenous factors such as oxygen concentration, metallic cations, visible light, amine oxidases, and spermatozoa also have important roles in ROS production. In preimplantation embryos, inhibition of oxidative phosphorylation (OXPHOS) reduces ROS production, which produces a positive effect in both porcine and bovine in vitro embryo development [27]. Manes and Lai [51] described the production of superoxide anions and H2O2 through NADPH oxidase on the rabbit blastocyst surface, whereas Nasr-Esfahani and Johnson [58] reported that an inhibitor of NADPH oxidase reduces H2O2 production in mouse embryos. In preimplantation embryo development, the end product of purine metabolism is xanthine [2], and inhibition of XO results in a low level of ROS in preimplantation embryos [58].
Oxygen concentration is also a factor in inducing ROS production. In monkey and rabbit oviducts, the O2 concentration is one-quarter to one-third of the atmospheric O2 concentration [48]. In in vitro culture medium at 37℃, the O2 concentration is considerably higher than the normal concentration of O2 within cells [92]. In vitro culture at the atmospheric O2 concentration elevates the ROS level in both mouse and bovine embryos, while a decreased O2 concentration (5%) increases embryo development in mouse [22]. Fischer and Bavister [19] reported that an increased O2 concentration during in vitro culture of embryos leads to increased ROS production, which adversely affects embryo development and quality. In addition, the exposure of embryos to visible light increases the production of ROS in embryos during in vitro development [56]. ROS production is increased at fertilization and at cell cleavage in bovine zygotes [46].
The ER is the first compartment of the secretory pathway in eukaryotic cells and is responsible for synthesis, modification, and delivery of proteins to the proper target site within the secretory pathway [21]. In the ER, a process termed quality control exports correctly folded proteins to the Golgi complex, while incompletely folded proteins are retained in the ER to complete the folding process [18]. The ERQC system is maintained by molecular chaperones, foldases, and lectins [72]. Proper folding of proteins or degradation of improperly folded proteins or folded polypeptides occurs in the ER, and ER-resident chaperones try to prevent aggregation of proteins through the ERQC system [17]. The ER is also the site for sterol and lipid synthesis [65]. When the ERQC system fails to respond, unfolded proteins accumulate in the ER [17].
The UPR includes different methods to re-establish protein synthesis, namely, translational attenuation to stop new protein entry into the ER, transcriptional activation of genes involved in protein folding, and transcriptional activation of genes encoding components of the ERAD system to decrease the number of misfolded proteins and inhibit activation of the apoptotic pathway to remove defective cells [432]. Several signaling pathways are involved in the UPR, which helps to maintain the normal physiological status of cells [1012]. The UPR is increased in preimplantation mouse embryos at the 8-cell, morula, and blastocyst stages [1]. ER transmembrane protein sensors are involved in maintaining cellular homeostasis, including inositol-requiring enzyme 1 (IRE1), double-stranded activated protein kinase-like ER kinase (PERK) [87], and activated transcription factor 6 (ATF6) [70], along with the ER molecular chaperone immunoglobulin-binding protein (BiP), also known as glucose-regulated protein 78 [6]. BiP, which belongs to the HSP70 family, is a stress-inducible chaperone [36]. BiP consists of three domains, a peptide-binding domain, an ATPase domain, and a C-terminal domain [38]. To prevent aggregation of unfolded proteins and promote proper folding, BiP binds to unfolded peptides through its peptide-binding domain and uses energy from ATP to promote proper folding [39]. BiP also maintains ER calcium homeostasis by binding to calcium, helping to immobilize calcium [44].
Under normal physiological conditions, BiP interacts with IRE1, PERK, and ATF6; however, when the level of misfolded/unfolded proteins increases, BiP is released from these inducers, leading to activation of the UPR [745]. Release of IRE1 from BiP induces non-conventional splicing of Xbp1 mRNA. Spliced Xbp1 (sXbp1) encodes a transcription factor that induces transcription of ER chaperones to maintain cellular homeostasis by participating in ER protein folding [23], whereas ATF6 cleavage is required for transcription of Xbp1 which subsequently undergoes IRE1 dependent splicing [84]. Attenuation of active Xbp1 protein expression in mouse embryos at the 2-cell stage improves embryo development until the blastocyst stage [94]. Hao et al. [28] reported that the UPR in mouse preimplantation embryos contributes to embryo death. Increased ER stress during preimplantation embryo development triggers the UPR, negatively affecting blastocyst formation and decreasing blastocyst development [696].
Mori and colleagues reported that IRE1-p is a bifunctional protein that possesses site-specific endoribonuclease (RNase) activity [54]. Two types of IRE1 have been identified, IRE1-alpha [81] and IRE1-beta [82]. The stress sensor IRE1-alpha is expressed in most cells and tissues [81]. The cleavage or splicing of IRE1-alpha and IRE1-beta are similar [59]. Suppression of IRE1-alpha causes embryonic lethality in mice [4467], whereas suppression of IRE1-beta has no phenotypic effect, and those mice were only susceptible to experimental intestinal colitis. Under normal conditions, IRE1 is bound to BiP and is in an inactive form [7]; however, an increased ROS level causes stress. Under such stress, IRE1 is released from BiP and changes to an active form [45] to control cellular homeostasis by participating in protein folding [39]. In cloned mammalian cells, overexpression of IRE1 activates BiP- and CHOP-encoding genes to promote cell death [72]. During bovine preimplantation embryo development, the activated form of IRE1 upregulates non-conventional Xbp1 splicing, activating transcriptional activator genes, which specifically results in the transcription of ER chaperones and the halting of embryo growth [75]. Inhibition of sXbp1 increases porcine embryo development and the expression of antiapoptotic genes, whereas the expression of proapoptotic genes decreases [95]. Moreover, inhibition of Xbp1 splicing in mouse preimplantation embryos reduces apoptosis and enhances embryo development [94]. Mori et al. [55], as well as Patil and Walter [65], reported that IRE1 is maintained in an inactive monomeric form due to its interaction with the protein chaperone BiP, whereas under ER stress conditions, IRE1-p is released from BiP and activates its RNase activity through homodimerization and trans-autophosphorylation, The RNase activity of IRE1-p cleaves a 252-base intron from the mRNA encoding the basic leucine zipper-containing transcription factor Hac1P; the spliced HAC1 mRNA encodes a protein that activates the transcriptional UPR element. Basar et al. [6] also investigated the role of BiP in preimplantation embryo development and found that the active form of BiP prevents blastocyst formation. During activation of the UPR, the IRE1 RNase cleaves Xbp1 mRNA to remove a 26-nucleotide intron, which creates a translational frame shift to generate a large form of Xbp1. This Xbp1 contains a novel transcriptional activation in its C-terminus [4373]. The sXbp1 acts as a transcriptional activator that has a major role in the activation of a wide variety of UPR target genes, which include some genes that require the IRE1/Xbp1 pathway and encode proteins that function in ERAD [164387]. These observations suggest that cells deficient in either IRE1 or Xbp1 are defective in ERAD [88] (Fig. 1).
Because ER stress responses are increased in eukaryotes containing additional sensors for the UPR, which promote stress adaptation or cell death in a more complex but coordinated manner, immediate transient attenuation of mRNA translation occurs in order to prevent continued influx of newly synthesized polypeptides into the stressed ER [36]. PERK is an ER transmembrane sensor protein present in the lumen of the ER and is attached to BiP. Upon accumulation of unfolded proteins in the ER lumen, dimerization of PERK released from BiP and trans-autophosphorylation lead to activation of the eukaryotic translational initiation factor 2 alpha (eIF2α) kinase function [28]. BiP plays a key role in proliferation and protection from apoptosis in the inner cell mass during early embryo development in mice [47]. PERK-mediated phosphorylation of eIF2α at ser51 initiates translation [25]. eIF2-α phosphorylation inhibits the guanine nucleotide exchange factor eIF2-β, which leads to inhibition of the exchange of guanosine diphosphate (GDP) with guanosine triphosphate (GTP) to form elF2-β-GTP-tRNA [30]. Aternary translation initiation complex is formed, which is required for AUG initiation codon recognition and joining of the 60S ribosomal subunit at the beginning of polypeptide chain synthesis. Translation initiation is dependent on the ternary complex, and a low level of ternary complex results in a low level of translation initiation [7]. The maximum level of UPR-dependent gene transcription is induced by PERK [66]. Activating transcription factor 4 (ATF4) is one of the basic transcription factors whose translation is initiated upon PERK-mediated phosphorylation of eIF2α (Fig. 1). PERK, eIF2α, and ATF4 phosphorylation are required for genes involved in amino acid biosynthesis and transport functions and the antioxidative stress response, as well as for genes that encode apoptosis-inducing factors such as growth arrest and DNA damage 34 and CHOP [83]. ROS enhance the expressions of ATF4 and CHOP, which negatively affect the development of bovine embryos [85]. The UPR in mouse cumulus-oocyte cells impairs embryo development [7883]. Phosphorylation of eIF2α mediates the majority of PERK signals, but nuclear factor erythroid-2 (NRF2) also mediates PERK signals [28]. NRF2 is attached to the microtubule-associated protein Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm. Under conditions that induce the UPR, PERK phosphorylates NRF2 to support its dissociation from Keap1. NRF2-knockout cells are sensitive to ER stress-induced apoptosis. PERK is required for cell survival, and NRF2 is a direct PERK substrate and PERK affecter [1415].
The ER membrane contains several cAMP response element-binding (CREB) proteins such as the CREB/ATF6 transcription factors, which regulate multiple UPR-related genes. The most well-known of these proteins is ATF6, which forms the third branch of the UPR, together with PERK and IRE1 [967]. Two homologous proteins, ATF6-alpha and ATF6-beta, are present in mammals [31]. BiP regulates ATF6 in a manner similar to the way it regulates PERK and IRE1 [74]. BiP regulates ATF6 through activation of two independent Golgi localization sequences, GLS1 and GLS2. BiP is attached to GLS1, but not to GLS2; thus, GLS1 is suppressed. However, BiP lacking GLS2 is dominant, which results in translocation of ATF6 to the Golgi complex and causes activation of ATF6 [6971]. Under normal conditions, ATF6 is in its resting form in the ER and is attached to BiP [8]. Upon unfolded protein accumulation, ATF6 is released from BiP and translocated to the Golgi complex [14]. In the Golgi complex, ATF6 is cleaved by site-1 and site-2 proteases [72]. These actions release the cytosolic N-terminal portion of ATF6 encoding a basic leucine zipper transcription factor [79] (Fig. 1). ATF6 binds to ER stress response elements 1 and 2 [38]. This binding requires nuclear factor alpha Y subunit and CCAAT-binding factor (NF-Y/CBF) [8690]. Important genes targeted by ATF6 are BiP, Xbp1, and CHOP/GADD153 during the UPR [8489]. In bovine preimplantation embryos, an increased ROS level enhances ATF6 expression, leading to upregulation of BiP, Xbp1, and CHOP, resulting in decreased blastocyst formation [5285]. A high level of palmitic acid in cumulus-oocyte complexes activates ATF6, negatively affecting mouse embryo development [83]. ATF6-alpha and ATF6-beta positively regulate transcription of ER-resident molecular chaperones and foldases, as shown by performing gene profiling analyses using overexpression of ATF6-alpha [5961] and ATF-beta [31]; however, the heterodimer complex of ATF6-alpha and ATF6-beta is a transcriptional repressor of the BiP promoter [7680].
In vitro fertilization and embryo development provide a unique window into embryonic cells, revealing cellular foundations that drive embryonic growth and development from the fertilized ovum into a competent blastocyst. A preimplantation embryo, while relatively simple in appearance, contains the cellular foundations to produce a highly complex mammalian organism. An increased ROS level in a preimplantation embryo leads to ER stress, which is involved in the etiology of defective embryo development. When ER stress exceeds the threshold of the UPR, apoptosis occurs. The adaptive response to ER stress, the UPR, is aimed at correcting overall protein processing in order to reduce the accumulation of misfolded proteins and restore normal cell function. If ER stress is severe or if the adaptive UPR fails, then cell death occurs. In the field of UPR research, further molecular research is required regarding the pathways that are activated after UPR signaling, especially in preimplantation embryos, because of the limited availability of previous reports. Additionally, further research is needed to determine whether one or several antioxidants should be used to detoxify ROS. Determining the most suitable medium for in vitro development of preimplantation embryos would provide the best possible start toward achieving the goal of an ROS-free environment. Further elucidation of new and relevant UPR signaling mechanisms in preimplantation embryos may provide useful clues regarding the regulation of normal embryonic development in vitro.
Figures and Tables
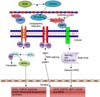 | Fig. 1Schematic diagram of in vitro developing cells exposed to an increased ROS level, inducing hypoxia and activating endoplasmic reticulum (ER) stress. During ER stress, immunoglobulin-binding protein (BiP) binds to misfolded proteins and ultimately activates three branches of the unfolded protein response (UPR): (1) protein kinase-like ER kinase (PERK); (2) inositol-requiring enzyme 1 alpha (IRE1α); and (3) activating transcription factor 6 (ATF6). PERK is activated by dimerization and autophosphorylation, leading to phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α), which activates transcription of ATF4, inducing the transcription of downstream genes. IRE1α produces a spliced form of Xbp1 due to its RNase activity. ATF6 is translocated from the ER to the Golgi body and is cleaved by protease activity to form active nuclear ATF6. |
Acknowledgments
This study was supported by The National Natural Science Foundation of China (project No. 31360546).
References
1. Abraham T, Pin CL, Watson AJ. Embryo collection induced transient activation of XBP1 arm of the ER stress response while embryo vitrification does not. Mol Hum Reprod. 2012; 18:229–242.

2. Alexiou M, Leese HJ. Purine utilization, de novo synthesis and degradation in mouse preimplantation embryos. Development. 1992; 114:185–192.

3. Ali AA, Bilodeau JF, Sirard MA. Antioxidant requirement for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology. 2003; 59:939–949.

4. Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012; 81:767–793.

5. Bain NT, Madan P, Betts DH. The early embryo response to intracellular reactive oxygen species is developmentally regulated. Reprod Fertil Dev. 2011; 23:561–575.

6. Basar M, Bozkurt I, Guzeloglu-Kayisli O, Sozen B, Tekmen I, Schatz F, Arici A, Lockwood CJ, Kayisli UA. Unfolded protein response prevents blastocyst formation during preimplantation embryo development in vitro. Fertil Steril. 2014; 102:1777–1784.

7. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000; 2:326–332.

8. Breckenridge DG, Germain M, Mathani JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003; 22:8608–8618.

9. Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AFG, Lavandero S. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol. 2013; 301:215–290.
10. Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum loaded to secretory capacity by processing the XBP-1 mRNA. Nature. 2002; 415:92–96.

11. Cebrian-Serrano A, Salvador I, Raga E, Dinnyes A, Silvestre MA. Beneficial effect of melatonin on blastocyst in vitro production from heat-stressed bovine oocytes. Reprod Domest Anim. 2013; 48:738–746.

12. Chakrabarti A, Chen AW, Varner JD. A review of the mammalian unfolded protein response. Biotechnol Bioeng. 2011; 108:2777–2793.

13. Chason RJ, Csokmay J, Segars JH, DeCherney AH, Armant DR. Environmental and epigenetic effects upon preimplantation embryo metabolism and development. Trends Endocrinol Metab. 2011; 22:412–420.

14. Chen X, Shen J, Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem. 2002; 277:13045–13052.

15. Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003; 23:7198–7209.

16. Díaz-Villanueva JF, Díaz-Molina R, García-González V. Protein folding and mechanisms of proteostasis. Int J Mol Sci. 2015; 16:17193–17230.

17. Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003; 4:181–191.

18. Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999; 286:1882–1888.

19. Fischer B, Bavister BD. Oxygen tension in oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993; 99:673–679.

20. Gardiner CS, Reed DJ. Synthesis of glutathione in the preimplantation mouse embryo. Arch Biochem Biophys. 1995; 318:30–36.

21. Gaut JR, Hendershot LM. The modification and assembly of proteins in the endoplasmic reticulum. Curr Opin Cell Biol. 1993; 5:589–595.

22. Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic Biol Med. 1993; 15:69–75.

23. Groenendyk J, Agellon LB, Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol. 2013; 75:49–67.

24. Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surrounding. Hum Reprod Update. 2001; 7:175–189.
25. Hall V, Hinichs K, Lazzari G, Betts DH, Hyttel P. Early embryonic development, assisted reproductive technologies, and pluripotent stem cell biology in domestic mammals. Vet J. 2013; 197:128–142.

26. Halliwell B. Antioxidant defense mechanisms: from the beginning to the end (of the beginning). Free Radic Res. 1999; 31:261–272.

27. Halliwell B, Gutteridge JMC. The chemistry of oxygen radicals and other oxygen-derived species. In : Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. 2nd ed. Oxford: Clarendon Press;1989. p. 22–85.
28. Hao L, Vassena R, Wu G, Han Z, Cheng Y, Latham KE, Sapienza C. The unfolded protein response contributes to preimplantation mouse embryo death in the DDK syndrome. Biol Reprod. 2009; 80:944–953.

29. Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002; 18:575–599.

30. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000; 5:897–904.

31. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003; 11:619–633.

32. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012; 13:89–102.

33. Houghton FD, Thompson JG, Kennedy CJ, Leese HJ. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev. 1996; 44:476–485.

34. Hubbard SC, Ivatt RJ. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981; 50:555–583.

35. Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994; 16:31–38.

36. Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000; 92:1564–1572.

37. Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002; 110:1389–1398.

38. King LS, Berg M, Chevalier M, Carey A, Elguindi EC, Blood SY. Isolation, expression and characterization of fully functional nontoxic BiP/GRP78 mutants. Protein Expr Purif. 2001; 22:148–158.

39. Kleizen B, Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004; 16:343–349.

40. Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985; 54:631–664.

41. Lane M, Gardner DK. Understanding cellular disruptions during early embryo development that perturb viability and fetal development. Reprod Fertil Dev. 2005; 17:371–378.

42. Lane M, Maybach JM, Gardner DK. Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Hum Reprod. 2002; 17:2686–2693.

43. Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003; 23:7448–7459.

44. Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002; 16:452–466.

45. Liu CY, Schröder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinase IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2000; 275:24881–24885.

46. Lopes AS, Lane M, Thompson JG. Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Hum Reprod. 2010; 25:2762–2773.

47. Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006; 26:5688–5697.

48. Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002; 318:1351–1365.

49. Maity P, Bindu S, Dey S, Goyal M, Alam A, Pal C, Reiter R, Bandyopadhyay U. Melatonin reduces indomethacin-induced gastric mucosal cell apoptosis by preventing mitochondrial oxidative stress and the activation of mitochondrial pathway of apoptosis. J Pineal Res. 2009; 46:314–323.

50. Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007; 18:716–731.

51. Manes C, Lai NC. Nonmitochondrial oxygen utilization by rabbit blastocysts and surface production of superoxide radicals. J Reprod Fertil. 1995; 104:69–75.

52. Michalak M, Gye MC. Endoplasmic reticulum stress in preiimplantation embryos. Clin Exp Reprod Med. 2015; 42:1–7.

53. Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol. 2010; 48:1105–1110.

54. Mori K, Ma W, Gething MJ, Sambrook JA. A transmembrane protein with a cdc2+/CDC 28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993; 74:743–756.

55. Mori K, Ogawa N, Kawahara T, Yanagi H, Yura T. mRNA splicing-mediated C- terminal replacement of transcription factor Hac1p is required for efficient activation of the unfolded protein response. Proc Natl Acad Sci U S A. 2000; 97:4660–4665.

56. Nakayama T, Noda Y, Goto Y, Mori T. Effects of visible light and other environmental factors on the production of oxygen radicals by hamster embryos. Theriogenology. 1994; 41:499–510.

57. Nasr-Esfahani MM, Aitken JR, Johnson MH. Hydrogen perioxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990; 109:501–507.

58. Nasr-Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development. 1991; 113:551–560.

59. Niwa M, Sidrauski C, Kaufman RJ, Walter P. A role of presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell. 1999; 99:691–702.

60. Ohlweiler LU, Burm DS, Leivas FG, Moyses AB, Ramos RS, Klein N, Mezzalira JC, Mezzalira A. Intracytoplasmic sperm injection improves in vitro embryo production from poor quality bovine oocytes. Theriogenology. 2013; 79:778–783.

61. Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002; 366:585–594.

62. Ou XH, Li S, Wang ZB, Li M, Quan S, Xing F, Guo L, Chao SB, Chen Z, Liang XW, Hou Y, Schatten H, Sun QY. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum Reprod. 2012; 27:2130–2145.

63. Overstrom EW, Duby RT, Dobrinsky J, Roche JF, Boland MP. Viability and oxidative metabolism of the bovine blastocyst. Theriogenology. 1992; 37:269.

64. Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003; 18:2270–2274.

65. Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001; 13:349–355.

66. Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006; 22:257–273.

67. Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, Horton HF, Scott A, Orkin SH, Byrne MC, Grusby MJ, Glimcher LH. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000; 14:152–157.

68. Reverendo M, Soares AR, Pereira PM, Carreto L, Ferreira M, Gatti E, Pierre P, Moura GR, Santos MA. TRNA mutations that effect decoding fidelity deregulate development and the proteostasis network in zebrafish. RNA Biol. 2014; 11:1199–1213.

69. Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002; 110:1383–1388.

70. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529.

71. Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001; 7:1165–1176.

72. Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005; 569:29–63.

73. Shah SZ, Zhao D, Khan SH, Yang L. Unfolded protein response pathways in neurodegenerative diseases. J Mol Neurosci. 2015; 57:529–637.

74. Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002; 3:99–111.

75. Succu S, Pasciu V, Manca ME, Celucci S, Torres-Rovira L, Leoni GG, Zinellu A, Carru C, Naitana S, Berlinguer F. Dose-dependent effect of melatonin on postwarming development of vitrified ovine embryos. Theriogenology. 2014; 81:1058–1066.

76. Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011; 13:184–190.

77. Tatone C, Di Emidio G, Vento M, Ciriminna R, Artini PG. Cryopreservation and oxidative stress in reproductive cells. Gynecol Endocrinol. 2010; 26:563–567.

78. Thompson JG, McNaughton C, Gasparrini B, McGowan LT, Tervit HR. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil. 2000; 118:47–55.

79. Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil. 1996; 106:299–306.

80. Thuerauf DJ, Morrison L, Glembotski CC. Opposing roles for ATF6α and ATF6β in endoplasmic reticulum stress response gene induction. J Biol Chem. 2004; 279:21078–21084.

81. Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998; 12:1812–1824.

82. Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998; 17:5708–5717.

83. Wu LL, Russel DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocytes complexes impairs pentraxin-3 sec secretion, mitochondrial membrane potential (ΔΨm), and embryo development. Mol Endocrinol. 2012; 26:562–573.

84. Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev Cell. 2007; 13:365–376.

85. Yoon SB, Choi SA, Sim BW, Kim JS, Mun SE, Jeong PS, Yang HJ, Lee Y, Park YH, Song BS, Kim YH, Jeong KJ, Huh JW, Lee SR, Kim SU, Chang KT. Developmental competence of bovine early embryos depends on the coupled response between oxidative and endoplasmic reticulum stress. Biol Reprod. 2014; 90:104.
86. Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998; 273:33741–33749.

87. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001; 107:881–891.

88. Yoshida H, Nadanaka S, Sato R, Mori K. XBP1 is critical to protect cells from endoplasmic reticulum stress: evidence from site-2 protease-deficient Chinese hamster ovary cells. Cell Struct Funct. 2006; 31:117–125.

89. Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000; 20:6755–6767.

90. Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factor 6α and 6β that activates the mammalian unfolded protein response. Mol Cell Biol. 2001; 21:1239–1248.

91. Yuan YQ, Van Soom A, Coopman FOJ, Mintiens K, Boerjan ML, Van Zeveren A, de Kruif A, Peelman LJ. Influence of oxygen tension on apoptosis and hatching in bovine embryos cultured in vitro. Theriogenology. 2003; 59:1585–1596.

92. Zhao HW, Haddad GG. Review: Hypoxic and oxidative stress resistance in Drosophila melanogaster. Placenta. 2011; 32:Suppl 2. S104–S108.
93. Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol. 2005; 283:40–57.

94. Zhang JY, Diao YF, Kim HR, Jin DI. Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLos One. 2012; 7:e40433.

95. Zhang JY, Diao YF, Oqani RK, Han RX, Jin DI. Effect of endoplasmic reticulum stress on porcine oocytes maturation and parthenogenetic embryonic development in vitro. Biol Reprod. 2012; 86:128.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download