Abstract
West Nile virus (WNV) is a mosquito-borne zoonotic pathogen that has spread throughout Europe and the United States. Recently, WNV spread to East and Southeast Asia, and great efforts have been made in South Korea to prevent the spread of WNV from neighboring countries. In this study, we diagnosed the first case of WNV in pigeons (Columba livia domestica) residing in cities using a competitive enzyme-linked immunosorbent assay and confirmed it with nested reverse transcription polymerase chain reaction analysis and sequencing. This is the first report to provide convincing evidence that WNV is present within South Korea.
West Nile virus (WNV) is spread by Culex spp. mosquitoes and propagated in birds, which are its natural host. Although humans and horses can be infected with WNV, they are end-point hosts and most infections are asymptomatic. In humans, approximately 20% of the infections present with mild headaches, myalgia, erythema and other symptoms, whereas meningitis and encephalitis are diagnosed in less than 1% of cases. The elderly are especially vulnerable to severe disease, often requiring hospitalization, with reported death rates of ~4% to 14%.
In 1990, WNV was identified as the causal agent of encephalitis in humans in Europe, highlighting its significance as a zoonotic pathogen [7]. The first case of WNV was reported in the United States in the summer of 1999. Because of the wide flyways of host migratory birds, the geographic range of WNV has been dramatically expanding for the last 15 years, which makes it one of the most widely spread arboviruses [2].
In South Korea, both the animal and plant quarantine agency (QIA) and Korea Centers for Disease Control and Prevention (KCDC) have been constantly monitoring migratory birds and mosquitoes; however, they have not reported any WNV cases in the country to date. Although there was one human case that was reported in South Korea in 2012, this case involved an individual infected in a foreign country before they returned home to South Korea [3].
Considering all of the rapid epidemiologic changes that have been continuously observed in Europe and the United States, we theorized that we should conduct surveillance programs focusing on domestic birds in South Korea for public health since it was possible that WNV could be introduced. In this study, we attempted to confirm the presence of WNV in domestic pigeons (Columba livia domestica) in South Korea using serological and molecular diagnostic techniques focusing on pigeons in areas in which the birds share territories with humans.
A total of 75 pigeons were captured, 25 birds each from the Northern region of Paju, the central region of Mungyeong and the Southern region of Busan in South Korea. All experimental procedures were performed in accordance with guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University. We used a competitive enzyme-linked immunosorbent assay (c-ELISAs) (IdVet ID Screen West Nile Competition; IdVet, France) to run serological tests on blood samples isolated from the captured pigeons. To detect WNV from tissue samples, we conducted nested reverse transcription polymerase chain reaction (RT-PCR) with WNV specific primers (primary primers, 1401: 5′-ACCAACTACTGTGGAGTC-3′, and 1845: 5′-TTCCAT CTTCACTCTACACT-3′; nested primers, 1485: 5′-GCCTTC ATACACACTAAAG-3′ and 1732: 5′-CCAATGCTATCACA GACT-3′) [4]. The PCR products were sent to Macrogen (Macrogen, Korea) for confirmation through sequencing analysis. A positive control was purchased from Sino Biological (WNV-prM/E cDNA Clone; Sino Biological, China).
Of the 75 pigeons, three (one each from the northern, central and southern regions of South Korea) tested positive for WNV envelop protein (E) by c-ELISA. In addition, the long term persistence of WNV with antibody was reported in wild birds 5.2 [9]. The pigeon that was captured from Busan had a high antibody titer, so we analyzed the corresponding tissue samples for the presence of WNV through PCR to confirm.
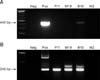
To investigate the specifications of WNV using PCR, we selected the E region of the genome, which encodes the envelop protein of WNV. The region is highly conserved in WNV and presents species-specific differences from other Flaviviridae, such as Japanese encephalitis virus and tick bone encephalitis [4]. As described in panel A in Fig. 1, primary RT-PCR results confirmed WNV in the tissue samples that were taken from the brains and kidneys of the positive bird from Busan, but not from the other two specimens. However, secondary nested PCR analysis showed the presence of WNV in the tissues of every sample (panel B in Fig. 1) that tested positive by c-ELISA.
Finally, in order to confirm the PCR results, we sequenced the isolated product (445 bp) and then compared the obtained sequences with the reference sequence of WNV from GenBank (National Center for Biotechnology Information, USA) using the Clustal W algorithm of the BioEdit Sequence Alignment Editor software (Ibis Biosciences, USA). Neighbor joining analysis with evolutionary distances calculated using the Tamura-Nei parameter model was conducted with MEGA version 5.2 [8]. Fig. 2 shows the phylogenetic relationships between various WNV species/genotypes and Korean isolates in this study. The Korean isolates (GenBank accession No. KU507578) were identified to cluster lineage 1. These findings add further evidence that the pigeons in South Korea are infected with WNV.
In addition, Japanese encephalitis virus (JEV) has IgM cross-reactivity with WNV in the c-ELISAs kit. Therefore, we checked the possibility of JEV infection or any other contamination in each sample. We cultured WNV using African green monkey kidney-derived Vero cells and repeated the entire process of detection without the positive sample. Based on the results, we could rule out the possibility of JEV infection. Although, we could not detect any WNV sequences other than the E region of WNV, the results clearly demonstrated the presence of WNV in Vero cells and supernatant based on the specific E region sequence. We assumed that the difficulty of additional sequence detection was caused by mutation of some WNV sequences; therefore, we plan to analyze the complete genome sequence using Next Generation Sequencing.
Cities are believed to the best place for pigeons to reproduce because of the abundance of good sources of food and nest sites. Pigeons are now well adapted to cities and share the territory with humans. Recently, there has been some concern about zoonoses that can be transmitted to humans. In particular, since pigeons co-exist with humans in cities, they are considered to be a high risk factor for transmitting avian-derived diseases because of the strong chance of contact with humans. Although WNV is technically not transmitted to humans from birds, since WNV spread to the United States in the 2000s, studies have been conducted to investigate the role pigeons in cities play in propagating and spreading WNV [5]. In addition, when a Eurasian color dove was experimentally infected, it was confirmed to have sufficient viremic levels to spread WNV [6]. The pigeons used in this study were captured in urban areas of northern, central and southern South Korea, where they are exposed to humans. South Korea is in danger of sustaining WNV, which presents a potential danger to people who might come into contact with infected birds.
The results of this study support the notion that WNV is already present within South Korea. Accordingly, South Korea should consider adopting an Early-Warning system, such as the WNV surveillance program that is currently in place in Greece, to prevent WNV infection [1]. Overall, this is the first study to investigate WNV in South Korea, and the results highlight the risk of WNV that is occurring in South Korea and the significance of further studies.
Figures and Tables
Fig. 1
Representative primary RT-PCR (A) and nested PCR (B) for West Nile viruses (WNV). WNV positive samples are indicated by a 445 bp band (primary RT-PCR) or a 248 bp band (nested PCR). Neg, negative control; Pos, positive control; P11, positive Paju sample 11; M19, positive Mungyeong sample 19; B10, positive Busan sample 10; M2, negative Mungyeong sample 2.
Acknowledgments
This study was partially supported by the Brain Korea 21 PLUS Program for Creative Veterinary Science Research, the Research Institute for Veterinary Science, Seoul National University, and the National Institute of Environmental Research (NIER) in Korea.
References
1. Chaintoutis SC, Dovas CI, Papanastassopoulou M, Gewehr S, Danis K, Beck C, Lecollinet S, Antalis V, Kalaitzopoulou S, Panagiotopoulos T, Mourelatos S, Zientara S, Papadopoulos O. Evaluation of a West Nile virus surveillance and early warning system in Greece, based on domestic pigeons. Comp Immunol Microbiol Infect Dis. 2014; 37:131–141.

2. Ciota AT, Kramer LD. Vector-virus interactions and transmission dynamics of West Nile virus. Viruses. 2013; 5:3021–3047.

3. Hwang J, Ryu HS, Kim H, Lee SA. The first reported case of West Nile encephalitis in Korea. J Korean Med Sci. 2015; 30:343–345.

4. Johnson DJ, Ostlund EN, Pedersen DD, Schmitt BJ. Detection of North American West Nile virus in animal tissue by a reverse transcription-nested polymerase chain reaction assay. Emerg Infect Dis. 2001; 7:739–741.

5. Komar N, Panella NA, Burns JE, Dusza SW, Mascarenhas TM, Talbot TO. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis. 2001; 7:621–625.

6. Panella NA, Young G, Komar N. Experimental infection of Eurasian collared-dove (Streptopelia decaocto) with West Nile virus. J Vector Ecol. 2013; 38:210–214.

7. Sambri V, Capobianchi M, Charrel R, Fyodorova M, Gaibani P, Gould E, Niedrig M, Papa A, Pierro A, Rossini G, Varani S, Vocale C, Landini MP. West Nile virus in Europe: emergence, epidemiology, diagnosis, treatment, and prevention. Clin Microbiol Infect. 2013; 19:699–704.

8. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28:2731–2739.

9. Wheeler SS, Vineyard MP, Woods LW, Reisen WK. Dynamics of West Nile virus persistence in House Sparrows (Passer domesticus). PLoS Negl Trop Dis. 2012; 6:e1860.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download