Abstract
Bovine embryos (day 5) were cultured to day 10 with or without 100 ng/mL PGF2α in medium supplemented with control; 100 nM Dex; 1,000 U/mL recombinant human leukemia inhibitory factor (rhLIF); or Dex+rhLIF. Although the rates to development to the blastocyst were not significantly different among groups, the hatching rate after additional culture with Dex +/or rhLIF was significantly higher in all supplemented groups than the control (p < 0.05). In the presence of PGF2α, the hatching rate was significantly restored in all supplemented groups relative to the group treated with only PGF2α and the control (p < 0.05). Embryo transfer (ET) was performed with blastocysts (day 7). PGF2α levels of control recipient cows were significantly higher in the circulatory blood samples collected 60 min after ET than in samples collected 60 min before ET (p < 0.005), and were decreased in cows injected with loading medium supplemented with Dex+rhLIF (p < 0.005). Pregnancy rate was significantly higher in the ET group that received supplemented embryo-loading medium than in the non-supplemented control (p < 0.05). The intrauterine administration of Dex and rhLIF at ET prevented increased PGF2α in circulatory blood and resulted in enhanced pregnancy rate.
Embryo transfer (ET) techniques, such as nonsurgical transfer/recovery and cryopreservation of embryos, superovulation, preparation of recipient animals, and in vitro production (IVP) of embryos, have developed into well-organized processes supporting many branches of the livestock industry, as well as biomedical science. However, some inefficiencies persist in ET, such as the rate of implantation and maintenance of pregnancy involving IVP-derived embryos [14]. Early embryonic loss within the first trimester of pregnancy has been considered one of the major problems affecting the ET industry in cattle [32]. Manipulation of the reproductive tracts of recipient cows during ET can result in an inflammatory environment within the uterine lumen, increasing the level of prostaglandin F2α (PGF2α) [182735]. This unusual elevation of endometrial PGF2α level during the peri-implantation period after ET reflects some similarities with luteolysis and is believed to have a negative effect on embryonic survival and quality, threatening the establishment of pregnancy [27]. Embryos cultured in medium supplemented with PGF2α have also shown impaired development in cattle, rabbits, and rats [2126].
Several recent studies have reported that supplementation with drugs or cytokines, such as glucocorticoids [24] and leukemia inhibitory factor (LIF) [4], can cause anti-inflammatory effects, alleviating actions against PGF2α and trophic effects in both embryos and endometrial cells in the cow. Glucocorticoids are known to be involved in various physiological processes, including general metabolism [37], female reproductive function [3], and anti-inflammatory actions, in various target organs [1016]. Dexamethasone (Dex), a synthetic glucocorticoid, is known to block the synthesis of prostaglandins, including PGF2α [33]. LIF, which is produced at the maternal-fetal interface, is a pleiotropic cytokine that has been shown to play an essential role in implantation in mice [11]. LIF is known to be involved in the proliferation and differentiation of trophoblasts, placentation, endometrial re-modeling, angiogenesis, and maternal tolerance of the conceptus in bovine species [2]. Therefore Dex, the synthetic inhibitor of PGF2α, and LIF, a cytokine associated with the establishment of implantation, may positively affect pregnancy rates in the presence of high levels of PGF2α after uterine manipulation [2738] in bovine ET. However, it is unclear if the administration of these additives can promote embryo survival and increase pregnancy rate after ET.
In the present study, we investigated the effects of Dex and recombinant human LIF (rhLIF) on in vitro embryo development in the presence of PGF2α and assessed in vivo levels of PGF2α before and after ET to determine if inflammation inhibitors play a role in controlling the amount of PGF2α released at the time of reproductive tract manipulation in the process of ET. We also examined the pregnancy rate in recipient cows after intrauterine administration of Dex and rhLIF.
All inorganic and organic compounds were purchased from Sigma-Aldrich Korea, unless indicated otherwise.
In vitro maturation (IVM) and in vitro fertilization (IVF) of oocytes and in vitro culture (IVC) of embryos was performed as described by Yamashita et al. [40]. Culture media (IVMD101 for IVM, IVF100 for IVF, and IVD101 for IVC) were purchased from the Research Institute for Functional Peptides (No. IFP3456K; Japan). Ovaries of Korean native cattle (Hanwoo) were collected from a local slaughterhouse and transported in saline at 25 to 35℃ to the laboratory within 2 to 3 h. Cumulus–oocyte complexes (COCs) were recovered by aspiration of 3 to 8 mm follicles using an 18-gauge hypodermic needle attached to a 10 mL disposable syringe and washed three times in HEPES-buffered Tyrode's medium. Next, COCs were cultured in 4-well culture dishes (Nalge Nunc International, USA) containing 500 µL of IVMD101 medium supplemented with 10 µL/mL follicle-stimulating hormone (FSH)-P (Folltrophin-V; Vertrepharm International, UK) and 10% fetal bovine serum (FBS; Gibco-BRL, USA) under warmed and gas-equilibrated mineral oil for 20 to 22 h at 38.5℃ under 5% CO2. The thawed sperm of Hanwoo were diluted with Sperm-Tyrode's albumin lactate pyruvate and prepared for IVF in IVF100 medium at a final concentration of 5.0 × 106/mL. After IVM, the matured oocytes were washed, transferred to IVF100 medium containing sperm, and incubated for 6 h at 38.5℃ in a humidified atmosphere of 5% CO2. Following IVF, the fertilized oocytes were transferred to IVD101 medium for five days, then transferred to CR2aa medium with 0.15% fatty acid-free bovine serum albumin (FAF-BSA), insulin-transferrin-selenium (Gibco-BRL), and 0.15% FBS at 39℃ in 5% CO2, 5% O2 and 90% N2 for another five days. For the ET experiment, fertilized oocytes were cultured in IVD101 medium for seven days under the same atmospheric conditions as above.
ET was carried out at a commercial Hanwoo farm located in Jeonnam Province, Korea. A total of 365 Korean native Hanwoo heifers were prepared as recipients. Before ET, the recipients were synchronized for estrus through administration of 25 mg of PGF2α (Lutalyse1; Pfizer, USA) on two occasions at a 14 day interval, then checked four times daily for behavioral signs of estrus. The recipients in the estrus were separated from the rest of the cattle for seven days, after which the corpus luteum (> 0.8 cm) was identified by rectal palpation. All embryos designated for ET were at the developmental stage (day 7) and of the highest quality grade (only grade 1) according to the Guide of the International Embryo Transfer Society [17]. On day 7, a single blastocyst was loaded into the medium designed through the pilot experiments and transferred nonsurgically to the uterine horn, ipsilateral to the corpus luteum of each recipient. For pregnancy diagnosis, recipients who did not return to estrus were initially selected, and pregnancies were confirmed by rectal palpation after 60 days. Pregnant cows were monitored by rectal palpation at regular intervals thereafter.
Blood samples were collected from recipient cows 1 h before and after ET by venipuncture of the median caudal vein or artery into tubes containing 100 µL of a 15% solution of EDTA (K3) (Tyco Healthcare Group, USA), then placed on ice until returning to the laboratory (< 4 h). Next, the blood was centrifuged at 1,500 × g for 10 min, after which the harvested plasma was stored at −20℃ until analysis. Duplicate serum aliquots were analyzed for each subject, and the average was used for statistical analysis. Plasma PGF2α concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits (Cayman Chemical, USA) according to the manufacturer's instructions. The coefficients of variation (CV) for the duplicates were less than 10% for all three markers tested, and inter-plate CV ranges were 5.8 to 6.2%.
Dex was dissolved in DMSO, and rhLIF (Millipore, USA) and PGF2α were dissolved in distilled water. For experiments, the reagents were dissolved in CR2aa culture medium with 0.15% FAF-BSA and 0.15% FBS. Prepared concentrations of the experimental agents were 100 ng/mL Dex, 1,000 U/mL rhLIF, and 100 ng/mL PGF2α.
In experiment 1, the effects of Dex and rhLIF on in vitro blastocyst formation we evaluated by transferring IVP-derived morulae (day 5) (17–20 embryos per group) to 20 µL of CR2aa medium with either Dex, rhLIF, Dex+rhLIF, or without Dex and rhLIF (control) and then culturing the samples until day 10. To evaluate the effects of PGF2α, PGF2α was added to the culture medium supplemented with Dex, rhLIF, or Dex+rhLIF. The rates of blastocyst development and hatching were then scored at day 7 and day 10, respectively. In experiment 2, the effects of intrauterine infusion of Dex and rhLIF on the level of PGF2α at embryo transfer were evaluated by measuring the PGF2α level in the blood plasma of recipient cows 60 min before and after embryo transfer. For intrauterine infusion of Dex and rhLIF, a 0.25 mL mini-straw for embryo transfer was inserted via the reproductive tract of recipient cows using an ET gun containing IVP blastocysts and the loading medium [TCM-199 (Gibco-BRL) + 10% FBS supplemented with Dex, rhLIF, Dex+rhLIF or no supplement control].
None of the experimental groups of morula-stage embryos cultured with Dex, rhLIF, or Dex+rhLIF displayed any differences from the control group with regard to the rate of blastocyst formation (panel A in Fig. 1) at Day 7. Moreover, PGF2α (100 ng/mL in CR2aa medium) did not cause any detrimental effects on blastocyst development at day 7 (panel B in Fig. 1). All experimental groups of morula-stage embryos cultured for an additional five days with Dex, rhLIF, or Dex+rhLIF (54.6%, 50.8%, and 56.1%, respectively) showed significantly higher (p < 0.005) blastocyst developmental than the control blastocysts (31.1%) (panel A in Fig. 2). The addition of PGF2α significantly decreased the hatching rate (12.1%) relative to that of the controls (27.1%). However, the hatching rate was significantly restored after supplementation with Dex, rhLIF, or Dex+rhLIF, (41.7%, 37.1%, and 28.8%, respectively) (p < 0.001) when compared with that of PGF2α only or the controls. No differences were identified between groups supplemented with Dex, rhLIF, or Dex + rhLIF in response to addition of PGF2α (panel B in Fig. 2).
The plasma concentration of PGF2α measured at 60 min after ET (74.80 ± 12.72, Mean ± SD) was significantly higher than that measured at 60 min before ET (282.93 ± 84.22, Mean ± SD, p < 0.005) (panel A in Fig. 3). Intrauterine infusion of Dex+rhLIF at the time of ET decreased the overall PGF2α concentration. There was no significant difference between the plasma level of PGF2α measured at 60 min before or after ET in experimental groups with Dex+rhLIF (96.6 ± 42.3 vs. 116.1 ± 42.6, Mean ± SD, p < 0.005) (panel B in Fig. 3). Intrauterine infusion of Dex and rhLIF at the time of ET significantly increased the overall pregnancy rate of recipient cows (n = 204) relative to the control animals (n = 161) (Table 1). The overall pregnancy rate of cows infused with Dex and rhLIF (71.1%) was significantly (p < 0.05) higher than that of control cows (59.0%).
The factors affecting the efficiency of ET include various technical and management problems [14]. It has been reported that manipulating the reproductive tract of recipient cows may cause elevated PGF2α levels during ET [182735]. The abnormal condition of elevated PGF2α at the time of implantation may be harmful to the corpus luteum of the recipient, creating a hostile uterine environment for implantation [27]. In addition, early embryos of several species have shown improper development when cultured in medium supplemented with PGF2α [2631]. In the present study, PGF2α was added to an embryo culture system to simulate the uterine environment immediately after ET, and Dex and rhLIF were supplemented to alleviate any inflammatory conditions. IVP morulae after compaction (day 5) were cultured for an additional five days in the PGF2α-supplemented medium, and the rate of development from the morula to the blastocyst was not affected by PGF2α when evaluated on day 7. However, addition of PGF2α to the culture medium significantly decreased the hatching rate of embryos, which was restored by Dex and rhLIF (p < 0.05) when evaluated on day 10. In the present study, the effects of each supplement on the developmental features of blastocysts were in accordance with those observed in previous studies [791324263031].
The addition of PGF2α has been shown to have a negative effect on overall bovine embryonic development, but embryos exposed in the later developmental stages have appeared to be less affected by PGF2α [212631]. Although gross findings of blastocysts were classified as having a normal morphology, their hatching rate, the key criteria determining the quality of embryos, was significantly negatively affected by PGF2α [212631]. The mechanism by which PGF2α influences embryonic development has remained unclear. PGF2α is related to altered cell-to-cell adhesion, induction of apoptosis, and altered Na+ transport within embryos [26]. These actions of PGF2α are thought to affect the maintenance of cavities or the expansion of blastocysts, thereby interfering with the hatching process. Once hatched, blastocysts continue to grow through elongation of the trophectoderm, which secretes interferon tau (IFN-tau) for attachment to the endometrial epithelium and for protection of the maternal corpus luteum from PGF2α [6]. In cows, the endometrial concentration of PGF2α is known to increase temporarily between days 16 and 19 of pregnancy, and the IFN-tau-secreting elongated embryos are more tolerant of PGF2α than blastocysts [28]. Therefore, it is reasonable to consider that precocious and accidental exposure to PGF2α can detrimentally affect the hatching process and the continuing development of blastocysts.
Although glucocorticoids are closely associated with the reproductive system, little is known about their effects on embryonic development. It has been reported that high doses of GCs negatively affect ovarian function, cell-cycle progression [9], oocyte maturation, and subsequent embryonic development in mice and pigs [93439]. However, several studies have shown differing results regarding the effects of GCs on oocytes and embryos depending on factors such as the origin of GCs, i.e., natural (corticosteroid) or synthetic (Dex), species differences, and culture systems. Moreover, Dex was recently shown to have no effect on oocyte maturation or subsequent embryonic development in mice, sheep and cows [13924]. Similar to the reports mentioned above, the present study showed that Dex added to the culture medium did not inhibit further development of blastocysts. The hatching rate in the Dex-supplemented group was increased relative to the control group, which corresponds with the finding that bovine embryos cultured with Dex tend to demonstrate a higher hatching rate than the control group [124]. Santana et al. [24] reported that expanded and hatched bovine blastocysts cultured with the addition of Dex presented significantly increased cell numbers, and the proliferative growth of blastocysts is assumed to positively influence the hatching process. GCs reportedly induce cell proliferation in somatic cells under stressful conditions via metabolic upregulation and act similarly in embryos during in vitro culture [1924]. However, further investigations are needed to clarify the role of GCs in embryonic development in vitro and in vivo, as well as in relation to stressful conditions.
LIF is a well-known cytokine in terms of its immune-modulatory, anti-inflammatory, and cell-proliferative roles in the implantation of several mammalian species [30]. However, the effects of LIF on embryonic development in in vitro culture systems have shown conflicting results depending on the composition of the medium [271330]. In the present study, the rate of developmental from morula to blastocyst was not affected by the addition of rhLIF to the culture medium; however, the hatching rate increased significantly compared to that of the control. These results correspond to those previously reported, in which supplementation of LIF influenced late embryonic development, especially hatching rate and total number of bovine embryos [71330]. Although the mechanism of the hatching process remains unclear, LIF appears to positively affect the hatching process by stimulating endogenous proteolytic activity or by enhancing the plasminogen activator of embryos through its proliferative effects [30].
The combination of Dex and rhLIF (Dex+rhLIF) increased the hatching rate of blastocysts cultured without PGF2α. Although there were no significant differences among treatment groups, the negative effects of PGF2α on the hatching process were more highly restored in blastocysts cultured in medium with only Dex added than in medium with only rhLIF or combined Dex and rhLIF. PGF2α has been shown to mediate apoptosis via direct or indirect pathways, which involves an increase in intracellular Ca2+ concentration through release from the endoplasmic reticulum [26] and formation of reactive oxygen species (ROS) [5]. Dex has been reported to inhibit apoptosis in various somatic cells, including bovine granulosa cells, and to stimulate apoptosis, depending on the extent of expression of anti-apoptotic genes, GC receptors, or cell type [2425]. However, few reports have been conducted to investigate the effects of GCs on embryonic development in vitro, and its mechanisms in apoptosis still remain unclear. However, dexamethasone may exhibit an anti-apoptotic function against the effects of PGF2α by enhancing cellular proliferation and the metabolic upregulation of blastomeres [192425]. The survival pathways driven by Dex may be regarded as eliciting a greater effect than the LIF-mediated processes. Both LIF and Dex in the present study were also assumed to coexist in different mechanisms involved in inter/intracellular communication and transport in blastocysts, as mentioned above. Additional studies are needed to investigate the effects and mechanisms of both Dex and LIF on late embryonic development and survival.
In the present study, the level of PGF2α significantly increased in the blood samples collected from control recipients 60 min after ET, similar to previous reports [18273538]. Dex and LIF were infused into the uterine lumen of recipients at the time of ET via loading medium containing embryos. Unlike our in vitro results, infusion of Dex and rhLIF was not associated with any differences compared to the control, (data not shown); however, the PGF2α level significantly decreased after infusion of Dex+rhLIF in recipients. The pregnancy rates in Dex+rhLIF-infused recipients were significantly higher than those in control recipients. The combination of Dex and rhLIF was thought to prevent excessive production of uterine PGF2α by the endometria of recipients. Biosynthesis of prostaglandins is mainly conducted by two types of cyclooxygenases (COX1 and COX2) present in endometrial cells. Cyclooxygenases convert endometrial arachidonic acid to the unstable PGG2, which is then converted to PGF and other PGs by endoperoxide isomerase (prostaglandin synthase) [22]. During manipulation of the reproductive tract, endometrial injuries may lead to an inflammatory situation and therefore the release of various inflammatory cytokines from the endometrium, such as tumor necrosis factor (TNF)-α, one of the most important cytokines, which is also known to mediate production of PGF2α from the endometrium at luteolysis [22]. The increased pregnancy rate of recipients in the present study supports the hypothesis that the level of PGF2α released from the endometrium at ET can be successfully suppressed by intrauterine administration of Dex+rhLIF.
Several trials and studies have investigated the inhibition of PGF2α synthesis during ET and at the time of implantation. PGF2α inhibitors mainly block essential enzymes for PGF2α synthesis, COX1 and COX2 [33]. Administration of flunixin meglumine [1227] and ibuprofen [15] in cows and aspirin [36] in humans improved pregnancy rates after ET. However, systematic and repeated administration of these nonsteroidal anti-inflammatory drugs (NSAIDs) may give rise to adverse side effects and residual problems [8].
Dex also blocks COX1 and COX2 [33] and, when compared to NSAIDs, has an extended elimination half-life (range, 36–72 h). This long-acting performance of Dex is advantageous for single-dose administration to inhibit pulsatile PGF2α. It has recently been suggested that low concentrations (10 nM to 100 µM/mL) of Dex can effectively suppress inflammatory cytokines (TNF-α, interleukin-1β and interleukin-6) from bovine alveolar macrophages [20]. The present study demonstrated that small doses and local administration of Dex can also suppress PGF2α secretion in the endometrium.
LIF is known to play important roles in embryo growth, implantation, and pregnancy in several species. However, the mechanism by which LIF functions at the very early stages of implantation in bovine species is still unclear. LIF gene expression can be measured at days 20–21 of pregnancy in the bovine inter-caruncular endometrium and increases at days 48–49 and 74–140 [23]. Recently, Al Naib et al. [2] suggested that LIF regulates the expression of NC4 (binding site of non-classical major histocompatibility complex [MHC] class I) in bovine blastocysts, which has previously been shown to induce human leukocyte antigen G (HLA-G) promoter activity cells [29]. LIF is also known to regulate the expression of the trophoblast-specific MHC class I protein, HLA-G, which is a non-classic class I MHC molecule that is specifically expressed at the time of uterine invasion and plays a role in facilitating immune tolerance of the conceptus [4]. Taken together, the improved pregnancy rate in the present study suggests that the synergetic effect of Dex and rhLIF can positively and locally affect the endometrial site of implantation and protect subsequent embryonic growth from PGF2α in recipient cows that received Dex and rhLIF.
In conclusion, the negative effects of endometrial PGF2α released at ET on embryonic development and pregnancy rate can be overcome by local intrauterine administration of Dex and rhLIF during embryo transfer. Further studies dealing with inhibitors of PGF2α and techniques to alleviate the release of PGF2α and reduce its effects are needed to improve the efficiency of ET.
Figures and Tables
 | Fig. 1Rate of blastocyst development from morula that developed from five to seven days in vitro and was cultured with Dex and rhLIF (A) or with PGF2α in the presence of Dex and rhLIF (B). The data represent the means ± SD of blastocysts from three independent replicated trials, using 72–150 (A) or 169–185 (B) embryos in each of the culture conditions. |
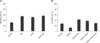 | Fig. 2Hatching rate of day 9 blastocysts cultured in vitro with Dex and rhLIF (A) or with PGF2α in the presence of Dex and rhLIF (B). Percentage of compacted embryos that continued development to hatched blastocysts after five to nine days of in vitro culture. The data represent the means ± SD of compete hatching of four (A) or three (B) independent replicated trials using 75–80 (A) or 92–102 (B) embryos for each culture condition. Asterisk indicates significant differences (p < 0.05). |
Acknowledgments
This study was supported by a grant from the Technology Development Program for Agriculture and Forestry, Ministry of Agriculture, Food and Rural Affairs [MAFRA; 111160-04, 111063032SB010 (-HD110)], Korea.
References
1. al-Hozab A, Menino AR Jr. Lack of effect of hormones and inducers of intracellular messengers on plasminogen activator production by bovine embryos in vitro. J Reprod Fertil. 1992; 96:79–90.

2. Al Naib A, Mamo S, O'Gormana GM, Lonergana P, Swalesb A, Fair T. Regulation of non-classical major histocompatability complex class I mRNA expression in bovine embryos. J Reprod Immunol. 2011; 91:31–40.

3. Andersen CY. Possible new mechanism of cortisol action in female reproductive organs: physiological implications of the free hormone hypothesis. J Endocrinol. 2002; 173:211–217.

4. Bamberger AM, Jenatschke S, Schulte HM, Löning T, Bamberger MC. Leukemia inhibitory factor (LIF) stimulates the human HLA-G promoter in JEG3 choriocarcinoma cells. J Clin Endocrinol Metab. 2000; 85:3932–3936.

5. Chaudhari N, Talwar P, Parimisetty A, Lefebvre d'Hellencourt C, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014; 8:213.

6. Dorniak P, Bazer FW, Spencer TE. Physiology and Endocrinology Symposium: biological role of interferon tau in endometrial function and conceptus elongation. J Anim Sci. 2013; 91:1627–1638.

7. Eswari S, Sai Kumar G, Sharma GT. Expression of mRNA encoding leukaemia inhibitory factor (LIF) and its receptor (LIFRβ) in buffalo preimplantation embryos produced in vitro: markers of successful embryo implantation. Zygote. 2013; 21:203–213.

8. Geary TW, Ansotegui RP, MacNeil MD, Roberts AJ, Waterman RC. Effects of flunixin meglumine on pregnancy establishment in beef cattle. J Anim Sci. 2010; 88:943–949.

9. González R, Ruiz-León Y, Gomendio M, Roldan ERS. The effect of glucocorticoids on mouse oocyte in vitro maturation and subsequent fertilization and embryo development. Toxicol In Vitro. 2010; 24:108–115.

10. Goppelt Struebe M. Molecular mechanisms involved in the regulation of prostaglandin biosynthesis by glucocorticoids. Biochem Pharmacol. 1997; 53:1389–1395.

11. Gough NM, Williams RL. The pleiotrophic actions of leukemia inhibitory factor. Cancer Cells. 1989; 1:77–80.
12. Guzeloglu A, Erdem H, Saribay MK, Thatcher WW, Tekeli T. Effect of the administration of flunixin meglumine on pregnancy rates in Holstein heifers. Vet Rec. 2007; 160:404–406.

13. Han YM, Lee ES, Mogoe T, Lee KK, Fukui Y. Effect of human leukemia inhibitory factor on in vitro development of IVF-derived bovine morulae and blastocysts. Theriogenology. 1995; 44:507–516.

14. Hasler JF. Forty years of embryo transfer in cattle: a review focusing on the journal Theriogenology, the growth of the industry in North America, and personal reminisces. Theriogenology. 2014; 81:152–169.

15. Heuwieser W, Iwersen M, Goetze L. Efficacy of carprofen on conception rates in lactating dairy cows after subcutaneous or intrauterine administration at the time of breeding. J Dairy Sci. 2011; 94:146–151.

16. Hillier SG, Tetsuka M. An anti-inflammatory role for glucocorticoids in the ovaries? J Reprod Immunol. 1998; 39:21–27.

17. International Embryo Transfer Society. Stringfellow DA, Seidel SM, editors. International Embryo Transfer Society. Manual of the International Embryo Transfer Society: a procedural guide and general information for the use of embryo transfer technology emphasizing sanitary procedures. Savoy: Manchester;1998. p. 103–134.
18. Kask K, Malmgren L, Odensvik K. Prostaglandin F2 alpha metabolite levels following an embryo transfer procedure in the mare. Acta Vet Scand. 1995; 36:145–147.

19. Krisher RL, Prather RS. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol Reprod Dev. 2012; 79:311–320.

20. Malazdrewich C, Thumbikat P, Abrahamsen MS, Maheswaran SK. Pharmacological inhibition of Mannheimia haemolytica lipopolysaccharide and leukotoxin-induced cytokine expression in bovine alveolar macrophages. Microb Pathog. 2004; 36:159–169.

21. Maurer PR, Beier HM. Uterine proteins and development in vitro of rabbit preimplantation embryos. J Reprod Fertil. 1976; 48:33–41.

22. Okuda K, Miyamoto Y, Skarzynski DJ. Regulation of endometrial prostaglandin F2α synthesis during luteolysis and early pregnancy in cattle. Domest Anim Endocrinol. 2002; 23:255–264.

23. Oshima K, Watanabe H, Yoshihara K, Kojima T, Dochi O, Takenouchi N, Fukushima M, Komatsu M. Gene expression of leukemia inhibitory factor (LIF) and macrophage colony stimulating factor (M-CSF) in bovine endometrium during early pregnancy. Theriogenology. 2003; 60:1217–1226.

24. Santana PPB, Carvalho CMF, da Costa NN, Silva TVG, Ramos PCA, Cordeiro MS, Santos SSD, Khayat AS, Ohashi OM, Miranda MS. Effect of dexamethasone on development of in vitro-produced bovine embryos. Theriogenology. 2014; 82:10–16.

25. Sasson R, Shinder V, Dantes A, Land A, Amsterdam A. Activation of multiple signal transduction pathways by glucocorticoids: protection of ovarian follicular cells against apoptosis. Biochem Biophys Res Commun. 2003; 311:1047–1056.

26. Scenna FN, Edwards JL, Rohrbach NR, Hockett ME, Saxton AM, Schrick FN. Detrimental effects of prostaglandin F2α on preimplantation bovine embryos. Prostaglandins Other Lipid Mediat. 2004; 73:215–226.

27. Scenna FN, Hockett ME, Townsa TM, Saxton AM, Rohrbach NR, Wehrman ME, Schrick FN. Influence of a prostaglandin synthesis inhibitor administered at embryo transfer on pregnancy rates of recipient cows. Prostaglandins Other Lipid Mediat. 2005; 78:38–45.

28. Seals RC, Lemaster JW, Hopkins FM, Schrick FN. Effects of elevated concentrations of prostaglandin F2α on pregnancy rates in progestogen supplemented cattle. Prostaglandins Other Lipid Mediat. 1998; 56:377–389.

29. Shido F, Ito T, Nomura S, Yamamoto E, Sumigama S, Ino K, Itakura A, Hattori A, Tsujimoto M, Mizutani S, Kikkawa F. Endoplasmic reticulum aminopeptidase-1 mediates leukemia inhibitory factor-induced cell surface human leukocyte antigen-G expression in JEG-3 choriocarcinoma cells. Endocrinology. 2006; 147:1780–1788.

30. Sirisathien S, Hernandez-Fonseca HJ, Bosch P, Hollet BR, Lott JD, Brackett BG. Effect of leukemia inhibitory factor on bovine embryos produced in vitro under chemically defined conditions. Theriogenology. 2003; 59:1751–1763.

31. Soto P, Natzke RP, Hansen PJ. Identification of possible mediators of embryonic mortality caused by mastitis: actions of lipopolysaccharide, prostaglandin F2α, and the nitric oxide generator, sodium nitroprusside dihydrate, on oocyte maturation and embryonic development in cattle. Am J Reprod Immunol. 2003; 50:263–272.

32. Taverne MAM, Breukelman SP, Perényi Ƶ, Dieleman SJ, Vos PLAM, Jonker HH, de Ruigh L, Van Wagtendonk-de Leeuw JM, Beckers JF. The monitoring of bovine pregnancies derived from transfer of in vitro produced embryos. Reprod Nutr Dev. 2002; 42:613–624.

33. Teeling JL, Cunningham C, Newman TA, Perry VH. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: implications for a role of COX-1. Brain Behav Immun. 2010; 24:409–419.

34. Van Merris V, Van Wemmel K, Cortvrindt R. In vitro effects of dexamethasone on mouse ovarian function and preimplantation embryo development. Reprod Toxicol. 2007; 23:32–41.

35. Velez JS, Randel RD, Neuendorff DA. Effect of uterine manipulation on postpartum fertility and plasma 13,14-dihydro-15-keto-prostaglandin F2α in Brahman cows and first-calf heifers. Theriogenology. 1991; 36:987–998.

36. Waldenström U, Hellberg D, Nilsson S. Low-dose aspirin in a short regimen as standard treatment in in vitro fertilization: a randomized, prospective study. Fertil Steril. 2004; 81:1560–1564.

37. Wang M. The role of glucocorticoid action in the pathophysiology of the metabolic syndrome. Nutr Metab (Lond). 2005; 2:3.
38. Wann RA, Randel RD. Effect of uterine manipulation 35 days after parturition on plasma concentrations of 13,14-dihydro-15-keto prostaglandin F2α in multiparous and primiparous Brahman cows. J Anim Sci. 1990; 68:1389–1394.

39. Yang JG, Chen WY, Li PS. Effects of glucocorticoids on maturation of pig oocytes and their subsequent fertilizing capacity in vitro. Biol Reprod. 1999; 60:929–936.

40. Yamashita S, Abe H, Itoh T, Satoh T, Hoshi H. A serum-free culture system for efficient in vitro production of bovine blastocysts with improved viability after freezing and thawing. Cytotechnology. 1999; 31:123–131.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download